The mammalian brain high-affinity L-proline transporter is enriched preferentially in synaptic vesicles in a subpopulation of excitatory nerve terminals in rat forebrain
- PMID: 9870934
- PMCID: PMC6782366
- DOI: 10.1523/JNEUROSCI.19-01-00021.1999
The mammalian brain high-affinity L-proline transporter is enriched preferentially in synaptic vesicles in a subpopulation of excitatory nerve terminals in rat forebrain
Abstract
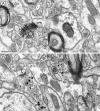
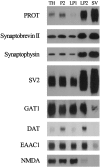
The expression of a brain-specific high-affinity Na+-dependent (and Cl--dependent) L-proline transporter (PROT) in subpopulations of putative glutamatergic neurons in mammalian brain suggests a physiological role for this carrier in excitatory neurotransmission (). To gain insights into potential sites where PROT may function, we used a C-terminal domain antipeptide antibody to determine the regional distribution and subcellular localization of PROT in rat forebrain. PROT immunoreactivity was seen in processes having a regional light microscopic distribution comparable to that of known glutamatergic projections within the cortex, caudate putamen nucleus (CPN), hippocampal formation, and other forebrain regions. In all regions examined by electron microscopy (cortex, CPN, and the stratum oriens of CA1), PROT labeling was observed primarily within subpopulations of axon terminals forming asymmetric excitatory-type synapses. Immunogold labeling for PROT was detected in close contact with membranes of small synaptic vesicles (SSVs) and more rarely with the plasma membrane in these axon terminals. Subcellular fractionation studies confirmed the preferential distribution of PROT to synaptic vesicles. The topology of PROT in synaptic vesicles was found to be inverted with respect to the plasma membrane, suggesting that PROT-containing vesicles are generated by a process involving endocytosis from the plasma membrane. Because PROT lacks any of the known characteristics of other vesicular transporters, these results suggest that certain excitatory terminals have a reserve pool of PROT associated with SSVs. The delivery of PROT to the plasma membrane by exocytosis could play a critical role in the plasticity of certain glutamatergic pathways.
Figures






References
-
- Alfonso A, Grundahl K, Duerr JS, Han HP, Rand JB. The Caenorhabditis elegans unc-17 gene: a putative vesicular acetylcholine transporter. Science. 1993;261:617–619. - PubMed
-
- Amara SG, Kuhar MJ. Neurotransmitter transporters: recent progress. Annu Rev Neurosci. 1993;16:73–93. - PubMed
-
- Balcar VJ, Johnston GAR, Stephenson AL. Transport of l-proline by rat brain slices. Brain Res. 1976;102:143–151. - PubMed
-
- Bennett JP, Logan WJ, Snyder SH. Amino acid neurotransmitter candidates: sodium-dependent high-affinity uptake by unique synaptosomal fractions. Science. 1972;178:997–999. - PubMed
-
- Birnbaum MJ. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989;57:305–315. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
