Heparin-binding epidermal growth factor-like growth factor in hippocampus: modulation of expression by seizures and anti-excitotoxic action
- PMID: 9870945
- PMCID: PMC6782387
- DOI: 10.1523/JNEUROSCI.19-01-00133.1999
Heparin-binding epidermal growth factor-like growth factor in hippocampus: modulation of expression by seizures and anti-excitotoxic action
Abstract
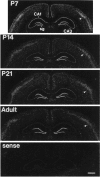
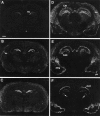
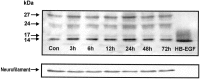
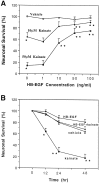
The expression of heparin-binding epidermal growth factor-like growth factor (HB-EGF), an EGF receptor ligand, was investigated in rat forebrain under basal conditions and after kainate-induced excitotoxic seizures. In addition, a potential neuroprotective role for HB-EGF was assessed in hippocampal cultures. In situ hybridization analysis of HB-EGF mRNA in developing rat hippocampus revealed its expression in all principle cell layers of hippocampus from birth to postnatal day (P) 7, whereas from P14 through adulthood, expression decreased in the pyramidal cell layer versus the dentate gyrus granule cells. After kainate-induced excitotoxic seizures, levels of HB-EGF mRNA increased markedly in the hippocampus, as well as in several other cortical and limbic forebrain regions. In the hippocampus, HB-EGF mRNA expression increased within 3 hr after kainate treatment, continued to increase until 24 hr, and then decreased; increases occurred in the dentate gyrus granule cells, in the molecular layer of the dentate gyrus, and in and around hippocampal pyramidal CA3 and CA1 neurons. At 48 hr after kainate treatment, HB-EGF mRNA remained elevated in vulnerable brain regions of the hippocampus and amygdaloid complex. Western blot analysis revealed increased levels of HB-EGF protein in the hippocampus after kainate administration, with a peak at 24 hr. Pretreatment of embryonic hippocampal cell cultures with HB-EGF protected neurons against kainate toxicity. The kainate-induced elevation of [Ca2+]i in hippocampal neurons was not altered in cultures pretreated with HB-EGF, suggesting an excitoprotective mechanism different from that of previously characterized excitoprotective growth factors. Taken together, these results suggest that HB-EGF may function as an endogenous neuroprotective agent after seizure-induced neural activity/injury.
Figures






References
-
- Abe K, Xie F-J, Saito H. Epidermal growth factor enhances short-term potentiation and facilitates induction of long-term potentiation in rat hippocampal slices. Brain Res. 1991;547:171–174. - PubMed
-
- Abe K, Ishiyama J, Saito H. Effects of epidermal growth factor and basic fibroblast growth factor on generation of long-term potentiation in the dentate gyrus of fimbria-fornix-lesioned rats. Brain Res. 1992;593:335–338. - PubMed
-
- Abraham JA, Damm D, Bajardi A, Miller J, Klagsbrun M, Ezekowitz AB. Heparin-binding EGF-like growth factor: characterization of rat and mouse cDNA clones, protein domain conservation across species, and transcript expression in tissues. Biochem Biophys Res Commun. 1993;190:125–133. - PubMed
-
- Alexi T, Hefti F. Trophic actions of transforming growth factor-α on mesencephalic dopaminergic neurons developing in culture. Neuroscience. 1993;55:903–918. - PubMed
-
- Barnard JA, Graves-Deal R, Pittelkow MR, DuBois R, Cook P, Ramsey GW, Bishop PR, Damstrup L, Coffey RJ. Auto- and cross-induction within the mammalian epidermal growth factor-related peptide family. J Biol Chem. 1994;269:22817–22822. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
