Bacterial filament formation, a defense mechanism against flagellate grazing, is growth rate controlled in bacteria of different phyla
- PMID: 9872755
- PMCID: PMC90978
- DOI: 10.1128/AEM.65.1.25-35.1999
Bacterial filament formation, a defense mechanism against flagellate grazing, is growth rate controlled in bacteria of different phyla
Abstract
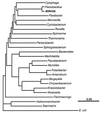
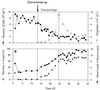
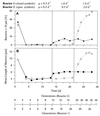
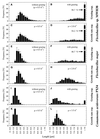
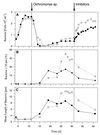
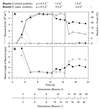
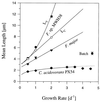
A facultatively filamentous bacterium was isolated from eutrophic lake water and was identified as Flectobacillus sp. strain MWH38 (a member of the Cytophaga-Flavobacterium-Bacteroides phylum) by comparative 16S rRNA gene sequence analysis. Filament formation by Flectobacillus sp. strain MWH38 and filament formation by Flectobacillus major, the closest known relative of strain MWH38, were studied in chemostat cultures under grazing pressure by the bacterivorous flagellate Ochromonas sp. strain DS and without predation at several growth rates. The results clearly demonstrated that filament formation by the two flectobacilli is growth rate controlled and thus independent of the presence of a predator. However, flagellate grazing positively influenced bacterial growth rates by decreasing bacterial biomass and thus indirectly stimulated filament formation. The results of investigations of cell elongation and filament formation by Comamonas acidovorans PX54 (a member of the beta subclass of the class Proteobacteria) supported the recent proposal that in this species the mechanism of filament formation is growth rate controlled. The finding that the grazing defense mechanism consisting of filament formation is growth rate controlled in the flectobacilli investigated and C. acidovorans PX54 (i.e., in bacteria belonging to divergent evolutionary phyla) may indicate that this mechanism is a phylogenetically widely distributed defense strategy against grazing.
Figures



References
-
- Bernardet J-F, Segers P, Vancanneyt M, Berthe F, Kersters K, Vandamme P. Cutting a Gordian knot: emended classification and description of the genus Flavobacterium, emended description of the family Flavobacteriaceae, and proposal of Flavobacterium hydatis nom. nov. (basonym, Cytophaga aquatilis Strohl and Tait 1978) Int J Syst Bacteriol. 1996;46:128–148.
-
- Berninger U-G, Finlay B F, Kuuppo-Leinikki P. Protozoan control of bacterial abundances in freshwater. Limnol Oceanogr. 1991;36:139–147.
-
- Chróst R J, Rai H. Bacterial secondary production. In: Overbeck J, Chróst R J, editors. Microbial ecology of Lake Plußsee. New York, N.Y: Springer; 1994. pp. 93–117.
-
- Chrzanowski T H, Šimek K. Prey-size selection by freshwater flagellated protozoa. Limnol Oceanogr. 1990;35:1424–1436.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

