Potential role of thiobacillus caldus in arsenopyrite bioleaching
- PMID: 9872756
- PMCID: PMC90979
- DOI: 10.1128/AEM.65.1.36-40.1999
Potential role of thiobacillus caldus in arsenopyrite bioleaching
Abstract
We investigated the potential role of the three strains of Thiobacillus caldus (KU, BC13, and C-SH12) in arsenopyrite leaching in combination with a moderately thermophilic iron oxidizer, Sulfobacillus thermosulfidooxidans. Pure cultures of T. caldus and S. thermosulfidooxidans were used as well as defined mixed cultures. By measuring released iron, tetrathionate, and sulfur concentrations, we found that the presence of T. caldus KU and BC13 in the defined mixed culture lowered the concentration of sulfur, and levels of tetrathionate were comparable to or lower than those in the presence of S. thermosulfidooxidans. This suggests that T. caldus grows on the sulfur compounds that build up during leaching, increasing the arsenopyrite-leaching efficiency. This result was similar to leaching arsenopyrite with a pure culture of S. thermosulfidooxidans in the presence of yeast extract. Therefore, three possible roles of T. caldus in the leaching environment can be hypothesized: to remove the buildup of solid sulfur that can cause an inhibitory layer on the surface of the mineral, to aid heterotrophic and mixotrophic growth by the release of organic chemicals, and to solubilize solid sulfur by the production of surface-active agents. The results showed that T. caldus KU was the most efficient at leaching arsenopyrite under the conditions tested, followed by BC13, and finally C-SH12.
Figures
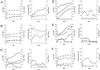
References
-
- Ahonen L, Tuovinen O H. Bacterial leaching of complex sulfide ore samples in bench-scale column reactors. Hydrometallurgy. 1995;37:1–21.
-
- Alexander B, Leach S, Ingledew W J. The relationship between chemiosmotic parameters and sensitivity to anions and organic acids in the acidophile Thiobacillus ferrooxidans. J Gen Microbiol. 1987;133:1171–1179.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

