In situ population dynamics of bacterial viruses in a terrestrial environment
- PMID: 9872776
- PMCID: PMC90999
- DOI: 10.1128/AEM.65.1.169-174.1999
In situ population dynamics of bacterial viruses in a terrestrial environment
Abstract
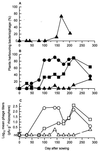
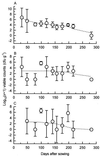
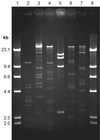
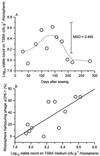
Predation by bacteriophages is thought to control bacterial numbers and facilitate gene transfer among bacteria in the biosphere. A thorough understanding of phage population dynamics is therefore necessary if their significance in natural environments is to be fully appreciated. Here we describe the in situ population dynamics of three separate phage populations predating on separate bacterial species, living on the surface of field-grown sugar beet (Beta vulgaris var. Amethyst), as recorded over a 9-month period. The distributions of the three phage populations were different and fluctuated temporally in 1996 (peak density, approximately 10(3) PFU g-1). One of these populations, predating on the indigenous phytosphere bacterium Serratia liquefaciens CP6, consisted of six genetically distinct DNA phages that varied in relative abundance to the extent that an apparent temporal succession was observed between the two most abundant phages, PhiCP6-1 and PhiCP6-4.
Figures

References
-
- Adams M H. Bacteriophages. New York, N.Y: Interscience Publishers; 1959.
-
- Bailey M J, Lilley A K, Thompson I P, Rainey P B, Ellis R J. Site directed chromosomal marking of a fluorescent pseudomonad isolated from the phytosphere of sugar beet; stability and potential for marker gene transfer. Mol Ecol. 1995;4:755–763. - PubMed
-
- Bergh Ø, Børsheim K Y, Bratbak G, Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989;340:467–468. - PubMed
-
- Chao L, Levin B R, Stewart F M. A complex community in a simple habitat: an experimental study with bacteria and phage. Ecology. 1977;58:369–378.
LinkOut - more resources
Full Text Sources

