Molecular epidemiology of Campylobacter jejuni in broiler flocks using randomly amplified polymorphic DNA-PCR and 23S rRNA-PCR and role of litter in its transmission
- PMID: 9872787
- PMCID: PMC91010
- DOI: 10.1128/AEM.65.1.260-263.1999
Molecular epidemiology of Campylobacter jejuni in broiler flocks using randomly amplified polymorphic DNA-PCR and 23S rRNA-PCR and role of litter in its transmission
Abstract
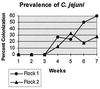
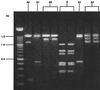
Poultry has long been cited as a reservoir for Campylobacter spp., and litter has been implicated as a vehicle in their transmission. Chicks were raised on litter removed from a broiler house positive for Campylobacter jejuni. Litter was removed from the house on days 0, 3, and 9 after birds were removed for slaughter. Chicks were raised on these three litters under controlled conditions in flocks of 25. None of these birds yielded C. jejuni in their cecal droppings through 7 weeks. Two successive flocks from the same Campylobacter-positive broiler house were monitored for Campylobacter colonization. Campylobacter jejuni prevalence rates were determined for each flock. Randomly amplified polymorphic DNA (RAPD)-PCR and 23S rRNA-PCR typing methods were used to group isolates. A high prevalence (60%) of C. jejuni in flock 1 coincided with the presence of an RAPD profile not appearing in flock 2, which had a lower rate of prevalence (28%). A 23S rRNA-PCR typing method was used to determine if strains with different RAPD profiles and different prevalence rates contained different 23S sequences. RAPD profiles detected with higher prevalence rates contained a spacer in the 23S rRNA region 100% of the time, while RAPD profiles found with lower prevalence rates contained an intervening sequence less than 2% of the time. Data suggest varying colonizing potentials of different RAPD profiles and a source other than previously used litter as a means of transmission of C. jejuni. These molecular typing methods demonstrate their usefulness, when used together, in this epidemiologic investigation.
Figures
References
-
- Annan-Prah S, Jane M. The mode of spread of Campylobacter jejuni/coli to broiler flocks. J Vet Med. 1988;35:11–18. - PubMed
-
- Barnhart H M, Dreesen D W, Bastien R, Pancorbo O C. Prevalence of Salmonella enteritidis and other serovars in ovaries of layer hens at time of slaughter. J Food Prot. 1991;54:448–491. - PubMed
-
- Berndtson E, Emanuelson U, Engvall A, Danielsson-Tham M L. A 1-year epidemiological study of campylobacters in 18 Swedish farms. Prev Vet Med. 1996;26:167–185.
-
- Beumer R R, de Vries J, Rombouts F M. Campylobacter jejuni non-culturable coccoid cells. Int J Food Microbiol. 1992;15:153–163. - PubMed
-
- Hernandez J, Fayos A, Ferrus M A, Owen R J. Random amplified polymorphic DNA fingerprinting of Campylobacter jejuni and C. coli isolated from human faeces, seawater and poultry products. Res Microbiol. 1995;146:685–696. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous