The alpha subunit of toluene dioxygenase from Pseudomonas putida F1 can accept electrons from reduced FerredoxinTOL but is catalytically inactive in the absence of the beta subunit
- PMID: 9872799
- PMCID: PMC91022
- DOI: 10.1128/AEM.65.1.315-318.1999
The alpha subunit of toluene dioxygenase from Pseudomonas putida F1 can accept electrons from reduced FerredoxinTOL but is catalytically inactive in the absence of the beta subunit
Abstract
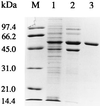
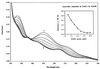
The oxygenase component of toluene dioxygenase from Pseudomonas putida F1 is an iron-sulfur protein (ISPTOL) consisting of alpha (TodC1) and beta (TodC2) subunits. Purified TodC1 gave absorbance and electron paramagnetic resonance spectra identical to those given by purified ISPTOL. TodC1 was reduced by NADH and catalytic amounts of ReductaseTOL and FerredoxinTOL. Reduced TodC1 did not oxidize toluene, and catalysis was strictly dependent on the presence of purified TodC2.
Figures
References
-
- Beinert H. Recent developments in the field of iron-sulfur proteins. FASEB J. 1990;4:2483–2491. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Butler C S, Mason J R. Structure-function analysis of the bacterial aromatic ring-hydroxylating dioxygenases. Adv Microb Physiol. 1997;38:47–84. - PubMed
-
- Cline J F, Hoffman B M, Mims W B, LaHaie E, Ballou D P, Fee J A. Evidence for N coordination to Fe in the [2Fe-2S] clusters of Thermus Rieske protein and phthalate dioxygenase from Pseudomonas. J Biol Chem. 1985;260:3251–3254. - PubMed
-
- Davis R W, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1980.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

