A T cell receptor-specific blockade of positive selection
- PMID: 9874560
- PMCID: PMC1887687
- DOI: 10.1084/jem.189.1.13
A T cell receptor-specific blockade of positive selection
Abstract
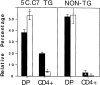
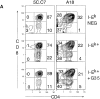
To investigate the influence of endogenous peptides on the developmental processes that occur during thymocyte selection, we have used monoclonal antibodies that preferentially recognize the major histocompatibility complex (MHC) molecule I-Ek when it is bound to the moth cytochrome c peptide (88-103). One of these antibodies (G35) specifically blocks the positive selection of transgenic thymocytes expressing a T cell receptor that is reactive to this peptide- MHC complex. Furthermore, G35 does not block the differentiation of transgenic T cells bearing receptors for a different I-Ek-peptide complex. This antibody recognizes a subset of endogenous I-Ek-peptide complexes found on a significant fraction of thymic antigen-presenting cells, including cortical and medullary epithelial cells. The sensitivity of G35 to minor alterations in peptide sequence suggests that the thymic peptide-MHC complexes that mediate the positive selection of a particular class II MHC-restricted thymocyte are structurally related to the complexes that can activate it in the periphery.
Figures









References
-
- Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Positive and negative selection of an antigen receptor on T cells in transgenic mice. Nature. 1988;336:73–76. - PubMed
-
- Kisielow P, Teh HS, Bluthmann H, von Boehmer H. Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature. 1988;335:730–723. - PubMed
-
- Berg LJ, Pullen AM, Fazekas de St B, Groth, Mathis D, Benoist C, Davis MM. Antigen/MHC-specific T cells are preferentially exported from the thymus in the presence of their MHC ligand. Cell. 1989;58:1035–1046. - PubMed
-
- Bevan MJ. In thymic selection, peptide diversity gives and takes away. Immunity. 1997;7:175–178. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

