Immune adherence-mediated opsonophagocytosis: the mechanism of Leishmania infection
- PMID: 9874561
- PMCID: PMC1887685
- DOI: 10.1084/jem.189.1.25
Immune adherence-mediated opsonophagocytosis: the mechanism of Leishmania infection
Abstract
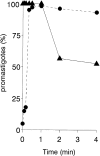
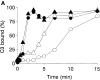
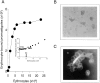
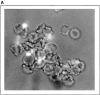
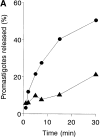
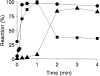
To mimic the sandfly pool feeding process and characterize the cellular and biochemical events that occur during the early stages of promastigote-host interaction, we developed an ex vivo model of human blood infection with Leishmania promastigotes. Within 30 s of blood contact, Leishmania promastigotes bind natural anti-Leishmania antibodies, which then activate the classical complement pathway and opsonization by the third component of complement. The opsonized promastigotes undergo an immune adherence reaction and bind quantitatively to erythrocyte CR1 receptors; opsonized Leishmania amastigotes also bind to erythrocytes. Progression of infection implies promastigote transfer from erythrocytes to acceptor blood leukocytes. After 10 min of ex vivo infection, 25% of all leukocytes contain intracellular parasites, indicating that blood cells are the early targets for the invading promastigotes. We propose that adaptation to the immune adherence mechanism aids Leishmania survival, promoting rapid promastigote phagocytosis by leukocytes. This facilitates host colonization and may represent the parasite's earliest survival strategy. In light of this mechanism, it is unlikely that infection-blocking vaccines can be developed.
Figures




Similar articles
-
Kinetic analysis of ex vivo human blood infection by Leishmania.PLoS Negl Trop Dis. 2010 Jul 13;4(7):e743. doi: 10.1371/journal.pntd.0000743. PLoS Negl Trop Dis. 2010. PMID: 20644618 Free PMC article.
-
Early mechanisms of Leishmania infection in human blood.Microbes Infect. 2003 May;5(6):507-13. doi: 10.1016/s1286-4579(03)00071-6. Microbes Infect. 2003. PMID: 12758280 Review.
-
Leishmania immune adherence reaction in vertebrates.Parasite Immunol. 2001 May;23(5):259-65. doi: 10.1046/j.1365-3024.2001.00380.x. Parasite Immunol. 2001. PMID: 11309136
-
Leishmania, macrophages and complement: a tale of subversion and exploitation.Parasitology. 1997;115 Suppl:S9-23. doi: 10.1017/s0031182097001789. Parasitology. 1997. PMID: 9571687 Review.
-
Growth cycle-dependent generation of complement-resistant Leishmania promastigotes.J Immunol. 1985 Apr;134(4):2713-8. J Immunol. 1985. PMID: 3973390
Cited by
-
Differential surface deposition of complement proteins on logarithmic and stationary phase Leishmania chagasi promastigotes.J Parasitol. 2012 Dec;98(6):1109-16. doi: 10.1645/GE-3130.1. Epub 2012 Jun 4. J Parasitol. 2012. PMID: 22662870 Free PMC article.
-
Transcriptional inhibition of interleukin-12 promoter activity in Leishmania spp.-infected macrophages.J Parasitol. 2008 Feb;94(1):84-93. doi: 10.1645/GE-1153.1. J Parasitol. 2008. PMID: 18372625 Free PMC article.
-
Role of Metallic Nanoparticles in Vaccinology: Implications for Infectious Disease Vaccine Development.Front Immunol. 2017 Mar 8;8:239. doi: 10.3389/fimmu.2017.00239. eCollection 2017. Front Immunol. 2017. PMID: 28337198 Free PMC article. Review.
-
The roles of complement receptor 3 and Fcγ receptors during Leishmania phagosome maturation.J Leukoc Biol. 2013 Jun;93(6):921-32. doi: 10.1189/jlb.0212086. Epub 2013 Mar 29. J Leukoc Biol. 2013. PMID: 23543768 Free PMC article.
-
Plasmodium falciparum uses a key functional site in complement receptor type-1 for invasion of human erythrocytes.Blood. 2011 Aug 18;118(7):1923-33. doi: 10.1182/blood-2011-03-341305. Epub 2011 Jun 17. Blood. 2011. PMID: 21685372 Free PMC article.
References
-
- Ribeiro JMC. Blood-feeding arthropods: live syringes or invertebrate pharmacologists? . Infect Agents Dis. 1995;4:143–152. - PubMed
-
- Mosser DM, Rosenthal LA. Leishmania–macrophage interactions: multiple receptors, multiple ligands and diverse cellular responses. Semin Cell Biol. 1993;4:315–322. - PubMed
-
- Brittingham A, Mosser DM. Exploitation of the complement system by Leishmaniapromastigotes. Parasitol Today. 1996;12:444–447. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

