DNA vaccination: transfection and activation of dendritic cells as key events for immunity
- PMID: 9874573
- PMCID: PMC1887690
- DOI: 10.1084/jem.189.1.169
DNA vaccination: transfection and activation of dendritic cells as key events for immunity
Abstract
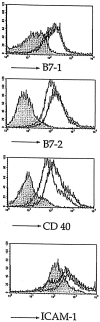
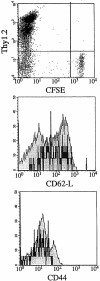
The mechanisms underlying initiation and maintenance of CD4 T cell responses after DNA vaccination were studied using a construct coding for nonsecreted fifth component of complement (C5) protein, thus restricting the availability of antigen. The only cell types to express C5 were keratinocytes at the site of DNA application and a small number of dendritic cells present in the draining lymph nodes. Antigen expression persisted for up to 12 wk in keratinocytes, but dendritic cells did not express C5 beyond 2 wk after vaccination. Cross-priming of dendritic cells by C5 expressed in keratinocytes did not occur unless keratinocyte death was induced by irradiation in vitro. CD4 T cells were activated in the draining lymph nodes only and subsequently migrated to the spleen, where memory T cells persisted for longer than 40 wk despite the absence of a source of persistent antigen. While DNA vaccination resulted in transfection of a small proportion of dendritic cells only, it led to general activation of all dendritic cells, thus providing optimal conditions for effective T cell activation and maintenance of memory.
Figures
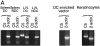
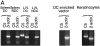
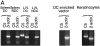
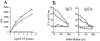

References
-
- Donnelly JJ, Ulmer B, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. - PubMed
-
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. - PubMed
-
- Condon C, Watkins SC, Celluzzi CM, Thompson K, Falo LD. DNA-based immunization by in vivo transfection of dendritic cells. Nat Med. 1996;2:1122–1128. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

