Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors
- PMID: 9874574
- PMCID: PMC1887693
- DOI: 10.1084/jem.189.1.179
Modulation of immune complex-induced inflammation in vivo by the coordinate expression of activation and inhibitory Fc receptors
Abstract
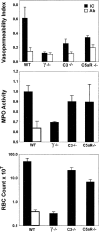
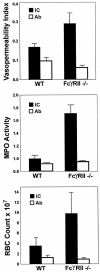
Autoantibodies and immune complexes are major pathogenic factors in autoimmune injury, responsible for initiation of the inflammatory cascade and its resulting tissue damage. This activation results from the interaction of immunoglobulin (Ig)G Fc receptors containing an activation motif (ITAM) with immune complexes (ICs) and cytotoxic autoantibodies which initiates and propagates an inflammatory response. In vitro, this pathway can be interrupted by coligation to FcgammaRIIB, an IgG Fc receptor containing an inhibitory motif (ITIM). In this report, we describe the in vivo consequences of FcgammaRII deficiency in the inflammatory response using a mouse model of IC alveolitis. At subthreshold concentrations of ICs that fail to elicit inflammatory responses in wild-type mice, FcgammaRII-deficient mice developed robust inflammatory responses characterized by increased hemorrhage, edema, and neutrophil infiltration. Bronchoalveolar fluids from FcgammaRII-/- stimulated mice contain higher levels of tumor necrosis factor and chemotactic activity, suggesting that FcgammaRII deficiency lowers the threshold of IC stimulation of resident cells such as the alveolar macrophage. In contrast, complement- and complement receptor-deficient mice develop normal inflammatory responses to suprathreshold levels of ICs, while FcRgamma-/- mice are completely protected from inflammatory injury. An inhibitory role for FcgammaRII on macrophages is demonstrated by analysis of FcgammaRII-/- macrophages which show greater phagocytic and calcium flux responses upon FcgammaRIII engagement. These data reveal contrasting roles for the cellular receptors for IgG on inflammatory cells, providing a regulatory mechanism for setting thresholds for IC sensitivity based on the ratio of ITIM to ITAM FcgammaR expression. Exploiting the FcgammaRII inhibitory pathway could thus provide a new therapeutic approach for modulating antibody-triggered inflammation.
Figures




References
-
- Ravetch JV, Clynes RA. Divergent roles for Fc receptors and complement in vivo. Annu Rev Immunol. 1998;16:421–432. - PubMed
-
- Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Adv Immunol. 1996;61:201–283. - PubMed
-
- Sylvestre DL, Ravetch JV. Fc receptors initiate the Arthus reaction: redefining the inflammatory cascade. Science. 1994;265:1095–1098. - PubMed
-
- Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

