Voltage-insensitive gating after charge-neutralizing mutations in the S4 segment of Shaker channels
- PMID: 9874694
- PMCID: PMC2222989
- DOI: 10.1085/jgp.113.1.139
Voltage-insensitive gating after charge-neutralizing mutations in the S4 segment of Shaker channels
Abstract
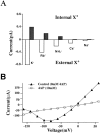
Shaker channel mutants, in which the first (R362), second (R365), and fourth (R371) basic residues in the S4 segment have been neutralized, are found to pass potassium currents with voltage-insensitive kinetics when expressed in Xenopus oocytes. Single channel recordings clarify that these channels continue to open and close from -160 to +80 mV with a constant opening probability (Po). Although Po is low ( approximately 0.15) in these mutants, mean open time is voltage independent and similar to that of control Shaker channels. Additionally, these mutant channels retain characteristic Shaker channel selectivity, sensitivity to block by 4-aminopyridine, and are partially blocked by external Ca2+ ions at very negative potentials. Furthermore, mean open time is approximately doubled, in both mutant channels and control Shaker channels, when Rb+ is substituted for K+ as the permeant ion species. Such strong similarities between mutant channels and control Shaker channels suggests that the pore region has not been substantially altered by the S4 charge neutralizations. We conclude that single channel kinetics in these mutants may indicate how Shaker channels would behave in the absence of voltage sensor input. Thus, mean open times appear primarily determined by voltage-insensitive transitions close to the open state rather than by voltage sensor movement, even in control, voltage-sensitive Shaker channels. By contrast, the low and voltage-insensitive Po seen in these mutant channels suggests that important determinants of normal channel opening derive from electrostatic coupling between S4 charges and the pore domain.
Figures




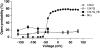

References
-
- Aggarwal SK, MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+channel. Neuron. 1996;16:1169–1177. - PubMed
-
- Armstrong CM. Sodium channels and gating currents. Physiol Rev. 1981;61:644–683. - PubMed
-
- Bao H, Hakeem A, McCormack K, Rayner M, Starkus J. Voltage-insensitive gating in ShakerB S4 mutants. Biophys J. 1996;70:A189.
-
- Bezanilla F, Stefani E. Voltage-dependent gating of ionic channels. Annu Rev Biophys Biomol Struct. 1994;23:819–846. - PubMed
-
- Catterall WA. Molecular properties of voltage-sensitive sodium channel. Annu Rev Biochem. 1986;55:953–985. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

