Influence of gender on prednisolone effects on whole blood T-cell deactivation and trafficking in rats
- PMID: 9874701
- PMCID: PMC4207271
- DOI: 10.1021/js9802695
Influence of gender on prednisolone effects on whole blood T-cell deactivation and trafficking in rats
Abstract
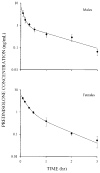
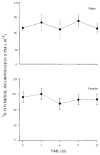
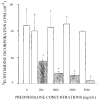
Prednisolone (5 mg/kg intravenous) was administered to adrenalectomized male and female Sprague-Dawley rats (250-350 g) to assess the effects of gender on disposition and pharmacoimmunodynamics. Plasma concentrations of prednisolone were determined by high-performance liquid chromatography. Incorporation of [3H]thymidine (3H-TDR) was used to determine whole blood T-cell (WBTC) trafficking and deactivation following stimulation with Concanavalin-A. Whole blood T-cell trafficking was determined indirectly by using the glucocorticoid receptor antagonist RU-40555 (250 ng/mL) added to ex vivo cultures of whole blood from animals dosed with prednisolone. Mean (+/- SD) prednisolone clearance values were 3.22 +/- 0.88 and 3.46 +/- 0.96 L/h/kg in males and females, respectively. After administration of prednisolone, relative T-cell counts decreased slowly with time to reach a nadir at 3-5 h and returned to baseline levels by 8 h. Fitting data using an indirect response model yielded mean prednisolone 50% inhibitory concentration for inhibition of WBTC trafficking (IC50T) that was lower in males compared with females (0.14 +/- 0.16 versus 1.03 +/- 0.06 ng/mL; p < 0.05). In the absence of RU-40555, an immediate and complete inhibition of 3H-TDR incorporation into WBTC was observed (deactivation) and baseline levels were recovered slowly as prednisolone was cleared from blood. The mean 50% inhibitory concentration for inhibition of WBTC deactivation (IC50D) based on an inhibitory Imax model was similar in males and females (0.20 +/- 0.24 versus 0.18 +/- 0.12 ng/mL). Although male and female rats have similar exposure to prednisolone after 5-mg/kg doses, males are more sensitive to the inhibition of WBTC trafficking, whereas no gender effects on deactivation of WBTC exist.
Figures

References
-
- Boumpas DT, Chousos GP, Wilder RL, Cupps TR, Balow JE. Glucocorticoid therapy for immune-mediated diseases: Basic and clinical correlates. Ann Intern Med. 1993;119:1198–1208. - PubMed
-
- Barnes PJ, Adcok I. Antiinflammatory actions of setroids: molecular mechanism. TiPS. 1993;14:436–441. - PubMed
-
- Fauci AS, Dale DC, Balow JE. Glucocorticoid therapy: mechanisms of action and clinical considerations. Ann Intern Med. 1976;84:304–315. - PubMed
-
- Abbas A, Lichman A, Pober J, editors. Cellular and molecular immunology. 2. W. B. Saunders; Philadelphia: 1994. Molecular basis of T-Cell antigen recognition and activation; p. 158.
-
- Crandall BG, Renlund DG, O’Connell JB. Increased cardiac allograft rejection in female heart transplant recipients. J Heart Transplant. 1988;7:419–423. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

