Pneumocysterol [(24Z)-ethylidenelanost-8-en-3beta-ol], a rare sterol detected in the opportunistic pathogen Pneumocystis carinii hominis: structural identity and chemical synthesis
- PMID: 9874778
- PMCID: PMC15099
- DOI: 10.1073/pnas.96.1.97
Pneumocysterol [(24Z)-ethylidenelanost-8-en-3beta-ol], a rare sterol detected in the opportunistic pathogen Pneumocystis carinii hominis: structural identity and chemical synthesis
Abstract
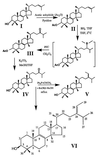
Pneumocystis carinii pneumonia (PcP) remains among the most prevalent opportunistic infections among AIDS patients. Currently, drugs used clinically for deep mycosis act by binding ergosterol or disrupting its biosynthesis. Although classified as a fungus, P. carinii lacks ergosterol. Instead, the pathogen synthesizes a number of distinct Delta7, 24-alkylsterols, despite the abundance of cholesterol, which it can scavenge from the lung alveolus. Thus, the pathogen-specific sterols appear vital for organism survival and proliferation. In the present study, high concentrations of a C32 sterol were found in human-derived P. carinii hominis. The definitive structural identities of two C-24 alkylated lanosterol compounds, previously not reported for rat-derived P. carinii carinii, were determined by using GLC, MS, and NMR spectroscopy together with the chemical syntheses of authentic standards. The C31 and C32 sterols were identified as euphorbol (24-methylenelanost-8-en-3beta-ol) and pneumocysterol [(24Z)-ethylidenelanost-8-en-3beta-ol], respectively. The identification of these and other 24-alkylsterols in P. carinii hominis suggests that (i) sterol C-24 methyltransferase activities are extraordinarily high in this organism, (ii) 24-alkylsterols are important components of the pathogen's membranes, because the addition of these side groups onto the sterol side chain requires substantial ATP equivalents, and (iii) the inefficacy of azole drugs against P. carinii can be explained by the ability of this organism to form 24-alkysterols before demethylation of the lanosterol nucleus. Because mammals cannot form 24-alkylsterols, their biosyntheses in P. carinii are attractive targets for the development of chemotherapeutic strategies against this opportunistic infection.
Figures


Similar articles
-
C27 to C32 sterols found in Pneumocystis, an opportunistic pathogen of immunocompromised mammals.Lipids. 2000 Mar;35(3):317-24. doi: 10.1007/s11745-000-0528-8. Lipids. 2000. PMID: 10783009
-
Sterol metabolism in the opportunistic pathogen Pneumocystis: advances and new insights.Lipids. 2004 Aug;39(8):753-61. doi: 10.1007/s11745-004-1292-5. Lipids. 2004. PMID: 15638243 Review.
-
Sterols of Pneumocystis carinii hominis organisms isolated from human lungs.Clin Diagn Lab Immunol. 1999 Nov;6(6):970-6. doi: 10.1128/CDLI.6.6.970-976.1999. Clin Diagn Lab Immunol. 1999. PMID: 10548595 Free PMC article.
-
Comprehensive and definitive structural identities of Pneumocystis carinii sterols.J Lipid Res. 2002 Jul;43(7):1114-24. doi: 10.1194/jlr.m200113-jlr200. J Lipid Res. 2002. PMID: 12091496
-
Is Pneumocystis a plant?J Eukaryot Microbiol. 2002 Sep-Oct;49(5):367-73. doi: 10.1111/j.1550-7408.2002.tb00214.x. J Eukaryot Microbiol. 2002. PMID: 12425523 Review.
Cited by
-
C27 to C32 sterols found in Pneumocystis, an opportunistic pathogen of immunocompromised mammals.Lipids. 2000 Mar;35(3):317-24. doi: 10.1007/s11745-000-0528-8. Lipids. 2000. PMID: 10783009
-
Sterol metabolism in the opportunistic pathogen Pneumocystis: advances and new insights.Lipids. 2004 Aug;39(8):753-61. doi: 10.1007/s11745-004-1292-5. Lipids. 2004. PMID: 15638243 Review.
-
Sterols of Pneumocystis carinii hominis organisms isolated from human lungs.Clin Diagn Lab Immunol. 1999 Nov;6(6):970-6. doi: 10.1128/CDLI.6.6.970-976.1999. Clin Diagn Lab Immunol. 1999. PMID: 10548595 Free PMC article.
-
Analysis of sphingolipids, sterols, and phospholipids in human pathogenic Cryptococcus strains.J Lipid Res. 2017 Oct;58(10):2017-2036. doi: 10.1194/jlr.M078600. Epub 2017 Aug 15. J Lipid Res. 2017. PMID: 28811322 Free PMC article.
-
Steroids from Marine-Derived Fungi: Evaluation of Antiproliferative and Antimicrobial Activities of Eburicol.Mar Drugs. 2019 Jun 21;17(6):372. doi: 10.3390/md17060372. Mar Drugs. 2019. PMID: 31234456 Free PMC article.
References
-
- Florin-Christensen M, Florin-Christensen J, Wu Y-P, Zhou L, Gupta A, Rudney H, Kaneshiro E S. Biochem Biophys Res Commun. 1993;198:236–242. - PubMed
-
- Kaneshiro E S, Ellis J E, Jayasimhulu K, Beach D H. J Eukaryotic Microbiol. 1994;41:78–85. - PubMed
-
- Haughan P A, Goad L J. In: Biochemical Protozoology. Coombs G H, North M D, editors. London: Taylor and Frances; 1991. pp. 312–328.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

