A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins
- PMID: 9874787
- PMCID: PMC15108
- DOI: 10.1073/pnas.96.1.151
A bioluminescence resonance energy transfer (BRET) system: application to interacting circadian clock proteins
Abstract
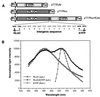
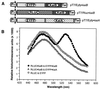
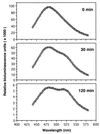
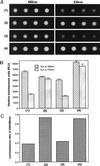
We describe a method for assaying protein interactions that offers some attractive advantages over previous assays. This method, called bioluminescence resonance energy transfer (BRET), uses a bioluminescent luciferase that is genetically fused to one candidate protein, and a green fluorescent protein mutant fused to another protein of interest. Interactions between the two fusion proteins can bring the luciferase and green fluorescent protein close enough for resonance energy transfer to occur, thus changing the color of the bioluminescent emission. By using proteins encoded by circadian (daily) clock genes from cyanobacteria, we use the BRET technique to demonstrate that the clock protein KaiB interacts to form homodimers. BRET should be particularly useful for testing protein interactions within native cells, especially with integral membrane proteins or proteins targeted to specific organelles.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

