Virus-specific immunity after gene therapy in a murine model of severe combined immunodeficiency
- PMID: 9874801
- PMCID: PMC15122
- DOI: 10.1073/pnas.96.1.232
Virus-specific immunity after gene therapy in a murine model of severe combined immunodeficiency
Abstract
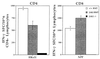
Human severe combined immunodeficiency (SCID) can be caused by defects in Janus kinase 3 (JAK3)-dependent cytokine signaling pathways. As a result, patients are at high risk of life-threatening infection. A JAK3 -/- SCID mouse model for the human disease has been used to test whether transplant with retrovirally transduced bone marrow (BM) cells (JAK3 BMT) could restore immunity to an influenza A virus. The immune responses also were compared directly with those for mice transplanted with wild-type BM (+/+ BMT). After infection, approximately 90% of the JAK3 BMT or +/+ BMT mice survived, whereas all of the JAK3 -/- mice died within 29 days. Normal levels of influenza-specific IgG were present in plasma from JAK3 BMT mice at 14 days after respiratory challenge, indicating restoration of B cell function. Influenza-specific CD4(+) and CD8(+) T cells were detected in the spleen and lymph nodes, and virus-specific CD8(+) effectors localized to the lungs of the JAK3 BMT mice. The kinetics of the specific host response correlated with complete clearance of the virus within 2 weeks of the initial exposure. By contrast, the JAK3 -/- mice did not show any evidence of viral immunity and were unable to control this viral pneumonia. Retroviral-mediated JAK3 gene transfer thus restores diverse aspects of cellular and humoral immunity and has obvious potential for human autologous BMT.
Figures






References
-
- Gurniak C B, Berg L J. Blood. 1996;87:3151–3160. - PubMed
-
- Ihle J N. Philos Trans R Soc London B. 1996;351:159–166. - PubMed
-
- Oakes S A, Candotti F, Johnston J A, Chen Y Q, Ryan J J, Taylor N, Liu X, Hennighausen L, Notarangelo L D, Paul W E, et al. Immunity. 1996;5:605–615. - PubMed
-
- Teglund S, McKay C, Schuetz E, van Deursen J M, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle J N. Cell. 1998;93:841–850. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

