Presynaptic effects of NMDA in cerebellar Purkinje cells and interneurons
- PMID: 9880571
- PMCID: PMC6782199
- DOI: 10.1523/JNEUROSCI.19-02-00511.1999
Presynaptic effects of NMDA in cerebellar Purkinje cells and interneurons
Abstract
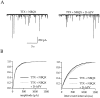
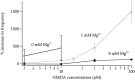
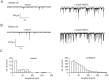
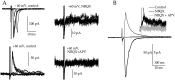
NMDA receptors (NMDARs) are generally believed to mediate exclusively postsynaptic effects at brain synapses. Here we searched for presynaptic effects of NMDA at inhibitory synapses in rat cerebellar slices. In Purkinje cells, application of NMDA enhanced the frequency of miniature IPSCs (mIPSCs) but not that of miniature EPSCs (mEPSCs). This increase in frequency was dependent on the external Mg2+ concentration. In basket and stellate cells, NMDA induced an even larger mIPSC frequency increase than in Purkinje cells, whereas mEPSCs were again not affected. Moreover, NMDA induced an inward current in both types of interneuron, which translated into a small depolarization (approximately 10 mV for 30 microM NMDA) under current-clamp conditions. In paired recordings of connected basket cell-Purkinje cell synapses, depolarizations of 10-30 mV applied to the basket cell soma enhanced the frequency of postsynaptic mIPSCs, suggesting that somatic depolarization was partially transmitted to the terminals in the presence of tetrodotoxin. However, this effect was small and unlikely to account fully for the effects of NMDA on mIPSCs. Consistent with a small number of dendritic NMDARs, evoked EPSCs in interneurons had a remarkably small NMDA component. Evoked IPSCs at interneuron-interneuron synapses were inhibited by NMDA, and the rate of failures was increased, indicating again a presynaptic site of action. We conclude that activation of NMDARs in interneurons exerts complex presynaptic effects, and that the corresponding receptors are most likely located in the axonal domain of the cell.
Figures




References
-
- Akazawa C, Shigemoto R, Bessho Y, Nakanishi S, Mizuno N. Differential expression of five N-methyl-d-aspartate receptor subunit mRNAs in the cerebellum of developing and adult rats. J Comp Neurol. 1994;347:150–160. - PubMed
-
- Berretta N, Jones RSG. Tonic facilitation of glutamate release by presynaptic N-methyl-d-aspartate autoreceptors in the entorhinal cortex. Neuroscience. 1996;75:339–344. - PubMed
-
- Bishop GA. An analysis of HRP-filled basket cell axons in the cat’s cerebellum. I. Morphometry and configuration. Anat Embryol. 1993;188:287–297. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
