Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode
- PMID: 9880580
- PMCID: PMC6782207
- DOI: 10.1523/JNEUROSCI.19-02-00599.1999
Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode
Abstract
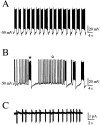
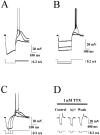
The modification of the discharge pattern of subthalamic nucleus (STN) neurons from single-spike activity to mixed burst-firing mode is one of the characteristics of parkinsonism in rat and primates. However, the mechanism of this process is not yet understood. Intrinsic firing patterns of STN neurons were examined in rat brain slices with intracellular and patch-clamp techniques. Almost half of the STN neurons that spontaneously discharged in the single-spike mode had the intrinsic property of switching to pure or mixed burst-firing mode when the membrane was hyperpolarized from -41.3 +/- 1.0 mV (range, -35 to -50 mV; n = 15) to -51.0 +/- 1.0 mV (range, -42 to -60 mV; n = 20). This switch was greatly facilitated by activation of metabotropic glutamate receptors with 1S,3R-ACPD. Recurrent membrane oscillations underlying burst-firing mode were endogenous and Ca2+-dependent because they were largely reduced by nifedipine (3 microM), Ni2+ (40 microM), and BAPTA-AM (10-50 microM) at any potential tested, whereas TTX (1 microM) had no effect. In contrast, simultaneous application of TEA (1 mM) and apamin (0.2 microM) prolonged burst duration. Moreover, in response to intracellular stimulation at hyperpolarized potentials, a plateau potential with a voltage and ionic basis similar to those of spontaneous bursts was recorded in 82% of the tested STN neurons, all of which displayed a low-threshold Ni2+-sensitive spike. We propose that recurrent membrane oscillations during bursts result from the sequential activation of T/R- and L-type Ca2+ currents, a Ca2+-activated inward current, and Ca2+-activated K+ currents.
Figures







References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of the basal ganglia disorders. Trends Neurosci. 1989;12:366–375. - PubMed
-
- Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl-4-phenyl-1,2,3–6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disord. 1991;6:288–292. - PubMed
-
- Bathia KP, Marsden CD. The behavioral and motor consequences of focal lesions of the basal ganglia in man. Brain. 1994;117:859–876. - PubMed
-
- Benazzouz A, Gross ChFéger J, Boraud Th, Bioulac B. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5:382–389. - PubMed
-
- Benazzouz A, Piallat B, Pollak P, Limousin P, Gao DM, Krack P, Benabid AL. Single unit recordings of subthalamic nucleus and pars reticulata of substantia nigra in akineto-rigid parkinsonian patients. Soc Neurosci Abstr. 1996;22:91.18.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
