Agonist-induced changes in substituted cysteine accessibility reveal dynamic extracellular structure of M3-M4 loop of glutamate receptor GluR6
- PMID: 9880585
- PMCID: PMC6782208
- DOI: 10.1523/JNEUROSCI.19-02-00644.1999
Agonist-induced changes in substituted cysteine accessibility reveal dynamic extracellular structure of M3-M4 loop of glutamate receptor GluR6
Abstract
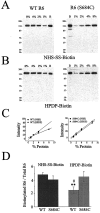
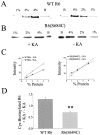
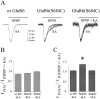
Recent evidence suggests that the transmembrane topology of ionotropic glutamate receptors differs from other members of the ligand-gated ion channel superfamily. However, the structure of the segment linking membrane domains M3 and M4 (the M3-M4 loop) remains controversial. Although various data indicate that this loop is extracellular, other results suggest that serine residues in this segment are sites of phosphorylation and channel modulation by intracellular protein kinases. To reconcile these data, we hypothesized that the M3-M4 loop structure is dynamic and, more specifically, that the portion containing putative phosphorylation sites may be translocated across the membrane to the cytoplasmic side during agonist binding. To test this hypothesis, we mutated Ser 684, a putative cAMP-dependent protein kinase site in the kainate-type glutamate receptor GluR6, to Cys. Results of biochemical and electrophysiological experiments are consistent with Cys 684 being accessible, in the unliganded state, from the extracellular side to modification by a Cys-specific biotinylating reagent followed by streptavidin (SA). Interestingly, our data suggest that this residue becomes inaccessible to the extracellular biotinylating reagent during agonist binding. However, we find it unlikely that Cys 684 undergoes membrane translocation, because the addition of SA to Cys-biotinylated GluR6(S684C) has no effect on peak glutamate-evoked current and only a small effect on macroscopic desensitization. We conclude that residue 684 in GluR6 is extracellular in the receptor-channel's closed, unliganded state and does not cross the membrane after agonist binding. However, an agonist-induced conformational change in the receptor substantially alters accessibility of position 684 to the extracellular environment.
Figures



References
-
- Bennett JA, Dingledine R. Topology profile for a glutamate receptor: three transmembrane domains and a channel-lining reentrant membrane loop. Neuron. 1995;14:3731–3784. - PubMed
-
- Chen N, Moshaver A, Raymond LA. Differential sensitivity of recombinant N-methyl-d-aspartate receptor subtypes to zinc inhibition. Mol Pharmacol. 1997;51:1015–1023. - PubMed
-
- Choi DW. Glutamate receptors and the induction of excitotoxic neuronal death. Prog Brain Res. 1994;100:47–51. - PubMed
-
- Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262:689–695. - PubMed
-
- Hall RA, Hansen A, Andersen PH, Soderling TR. Surface expression of the AMPA receptor subunits GluR1, GluR2, and GluR4 in stably transfected baby hamster kidney cells. J Neurochem. 1997;68:625–630. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
