A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent, JNK-independent induction of apoptosis by cytosine arabinoside
- PMID: 9880587
- PMCID: PMC6782192
- DOI: 10.1523/JNEUROSCI.19-02-00664.1999
A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent, JNK-independent induction of apoptosis by cytosine arabinoside
Abstract
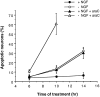
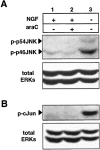
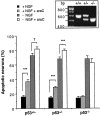
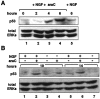
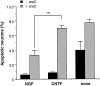
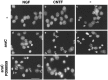
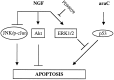
The antimitotic nucleoside cytosine arabinoside (araC) causes apoptosis in postmitotic neurons for which two mechanisms have been suggested: (1) araC directly inhibits a trophic factor-maintained signaling pathway required for survival, effectively mimicking trophic factor withdrawal; and (2) araC induces apoptosis by a p53-dependent mechanism distinct from trophic factor withdrawal. In rat sympathetic neurons, we found that araC treatment for 12 hr induced approximately 25% apoptosis without affecting NGF-maintained signaling; there was neither reduction in the activity of mitogen activated protein kinase/extracellular signal-regulated kinase (MAPK/ERK) or protein kinase B/Akt, a kinase implicated in NGF-mediated survival, nor was there c-Jun N-terminal kinase (JNK) activation or c-Jun N-terminal phosphorylation, events implicated in apoptosis induced by NGF withdrawal. However, araC treatment, but not NGF-withdrawal, elevated expression of p53 protein before and during apoptosis. Additionally, araC-induced apoptosis was suppressed in sympathetic neurons from p53 null mice. Although MAPK/ERK activity is not necessary for NGF-induced survival, it protected against toxicity by araC, because inhibition of the MAPK pathway by PD98059 resulted in a significant increase in the rate of apoptosis induced by araC in the presence of NGF. Consistent with this finding, ciliary neurotrophic factor, which does not cause sustained activation of MAPK/ERK, did not protect against araC toxicity. Our data show that, in contrast to NGF deprivation, araC induces apoptosis via a p53-dependent, JNK-independent mechanism, against which MAPK/ERK plays a substantial protective role. Thus, NGF can suppress apoptotic mechanisms in addition to those caused by its own deprivation.
Figures


References
-
- Baker WJ, Royer GLJ, Weiss RB. Cytarabine and neurologic toxicity. J Clin Oncol. 1991;9:679–693. - PubMed
-
- Buckmaster A, Nobes CD, Edwards SN, Tolkovsky AM. NGF is required for induction of c-fos immunoreactivity by serum, depolarisation, cyclic AMP or trauma in cultured rat sympathetic neurons. Eur J Neurosci. 1991;3:698–707. - PubMed
-
- Clarke AR, Purdie CA, Harrison DJ, Morris RG, Bird CC, Hooper ML, Wyllie AH. Thymocyte apoptosis induced by p53-dependent and independent pathways. Nature. 1993;362:849–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
