Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses
- PMID: 9880593
- PMCID: PMC6782194
- DOI: 10.1523/JNEUROSCI.19-02-00726.1999
Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses
Abstract
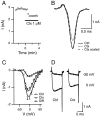
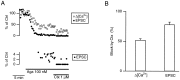
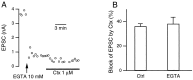
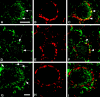
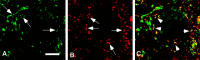
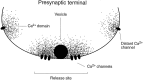
We studied how Ca2+ influx through different subtypes of Ca2+ channels couples to release at a calyx-type terminal in the rat medial nucleus of the trapezoid body by simultaneously measuring the presynaptic Ca2+ influx evoked by a single action potential and the EPSC. Application of subtype-specific toxins showed that Ca2+ channels of the P/Q-, N-, and R-type controlled glutamate release at a single terminal. The Ca2+ influx through the P/Q-type channels triggered release more effectively than Ca2+ influx through N- or R-type channels. We investigated mechanisms that contributed to these differences in effectiveness. Electrophysiological experiments suggested that individual release sites were controlled by all three subtypes of Ca2+ channels. Immunocytochemical staining indicated, however, that a substantial fraction of N- and R-type channels was located distant from release sites. Although these distant channels contributed to the Ca2+ influx into the terminal, they may not contribute to release. Taken together, the results suggest that the Ca2+ influx into the calyx via N- and R-type channels triggers release less effectively than that via P/Q-type because a substantial fraction of the N- and R-type channels in the calyx is localized distant from release sites.
Figures


References
-
- Artalejo CR, Adams ME, Fox AP. Three types of Ca2+ channel trigger secretion with different efficacies in chromaffin cells. Nature. 1994;367:72–76. - PubMed
-
- Augustine GJ, Adler EM, Charlton MP. The calcium signal for transmitter secretion from presynaptic nerve terminals. Ann NY Acad Sci. 1991;635:365–381. - PubMed
-
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
