ATP stimulates sympathetic transmitter release via presynaptic P2X purinoceptors
- PMID: 9880594
- PMCID: PMC6782222
- DOI: 10.1523/JNEUROSCI.19-02-00737.1999
ATP stimulates sympathetic transmitter release via presynaptic P2X purinoceptors
Abstract
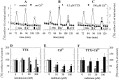
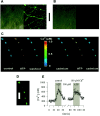
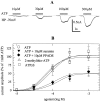
ATP is a fast transmitter in sympathetic ganglia and at the sympathoeffector junction. In primary cultures of dissociated rat superior cervical ganglion neurons, ATP elicits noradrenaline release in an entirely Ca2+-dependent manner. Nevertheless, ATP-evoked noradrenaline release was only partially reduced (by approximately 50%) when either Na+ or Ca2+ channels were blocked, which indicates that ATP receptors themselves mediated transmembrane Ca2+ entry. An "axonal" preparation was obtained by removing ganglia from explant cultures, which left a network of neurites behind; immunostaining for axonal and dendritic markers revealed that all of these neurites were axons. In this preparation, ATP raised intraaxonal Ca2+ and triggered noradrenaline release, and these actions were not altered when Ca2+ channels were blocked by Cd2+. Hence, Ca2+-permeable ATP-gated ion channels, i.e., P2X purinoceptors, are located at presynaptic sites and directly mediate Ca2+-dependent transmitter release. These presynaptic P2X receptors displayed a rank order of agonist potency of ATP >/= 2-methylthio-ATP > ATPgammaS >> alpha,beta-methylene-ATP approximately beta,gamma-methylene-L-ATP and were blocked by suramin or PPADS. ATP, 2-methylthio-ATP, and ATPgammaS also evoked inward currents measured at neuronal somata, but there these agonists were equipotent. Hence, presynaptic P2X receptors resemble the cloned P2X2 subtype, but they appear to differ from somatodendritic P2X receptors in terms of agonist sensitivity. Suramin reduced depolarization-evoked noradrenaline release by up to 20%, when autoinhibitory mechanisms were inactivated by pertussis toxin. These results indicate that presynaptic P2X purinoceptors mediate a positive, whereas G-protein-coupled P2Y purinoceptors mediate a negative, feedback modulation of sympathetic transmitter release.
Figures



References
-
- Augustine GJ, Neher E. Neuronal Ca2+ signalling takes the local route. Curr Opin Neurobiol. 1992;2:302–307. - PubMed
-
- Boehm S. Noradrenaline release from rat sympathetic neurons evoked by P2-purinoceptor activation. Naunyn Schmiedebergs Arch Pharmacol. 1994;350:454–458. - PubMed
-
- Boehm S, Huck S. α2-Adrenoceptor mediated inhibition of acetylcholine-induced noradrenaline release from rat sympathetic neurons: an action at voltage-gated Ca2+ channels. Neuroscience. 1995;69:221–231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
