Identification and characterization of early glial progenitors using a transgenic selection strategy
- PMID: 9880596
- PMCID: PMC6782214
- DOI: 10.1523/JNEUROSCI.19-02-00759.1999
Identification and characterization of early glial progenitors using a transgenic selection strategy
Abstract
To define the spatiotemporal development of and simultaneously select for oligodendrocytes (OLs) and Schwann cells (SCs), transgenic mice were generated that expressed a bacterial beta-galactosidase (beta-gal) and neomycin phosphotransferase fusion protein (betageo) under the control of murine 2'3'-cyclic nucleotide 3'-phosphodiesterase (muCNP) promoters I and II. Transgenic beta-gal activity was detected at embryonic day 12.5 in the ventral region of the rhombencephalon and spinal cord and in the neural crest. When cells from the rhombencephalon were cultured in the presence of G418, surviving cells differentiated into OLs, indicating that during development this brain region provides one source of OL progenitors. Postnatally, robust beta-gal activity was localized to OLs throughout the brain and was absent from astrocytes, neurons, and microglia or monocytes. In the sciatic nerve beta-gal activity was localized exclusively to SCs. Cultures from postnatal day 10 brain or sciatic nerve were grown in the presence of G418, and within 8-9 d exposure to antibiotic, 99% of all surviving cells were beta-gal-positive OLs or SCs. These studies demonstrate that the muCNP-betageo transgenic mice are useful for identifying OLs and SCs beginning at early stages of the glial cell lineage and throughout their development. This novel approach definitively establishes that the beta-gal-positive cells identified in vivo are glial progenitors, as defined by their ability to survive antibiotic selection and differentiate into OLs or SCs in vitro. Moreover, this experimental paradigm facilitates the rapid and efficient selection of pure populations of mouse OLs and SCs and further underscores the use of cell-specific promoters in the purification of distinct cell types.
Figures





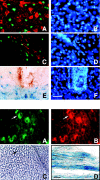

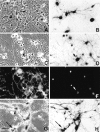

References
-
- Abney ER, Williams BP, Raff MC. Tracing the development of oligodendrocytes from precursor cells using monoclonal antibodies, fluorescence-activated cell sorting and cell culture. Dev Biol. 1983;100:166–171. - PubMed
-
- Bachmair A, Finley D, Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986;234:179–186. - PubMed
-
- Barres BA, Chun LLY, Corey DP. Ion channel in vertebrate glia. Annu Rev Neurosci. 1988;13:441–474. - PubMed
-
- Barres BA, Hart IK, Coles HSR, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. - PubMed
-
- Bernier L, Alvarez F, Norgard EM, Raible DW, Mentaberry A, Schembri JG, Sabatini DD, Colman DR. Molecular cloning of a 2′,3′-cyclic nucleotide 3′-phosphodiesterase: mRNAs with different 5′-ends encode the same set of proteins in nervous and lymphoid tissues. J Neurosci. 1987;7:2703–2710. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
