Tenascin-R inhibits the growth of optic fibers in vitro but is rapidly eliminated during nerve regeneration in the salamander Pleurodeles waltl
- PMID: 9880601
- PMCID: PMC6782211
- DOI: 10.1523/JNEUROSCI.19-02-00813.1999
Tenascin-R inhibits the growth of optic fibers in vitro but is rapidly eliminated during nerve regeneration in the salamander Pleurodeles waltl
Abstract
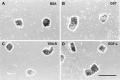
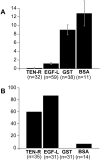
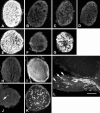
Tenascin-R is a multidomain molecule of the extracellular matrix in the CNS with neurite outgrowth inhibitory functions. Despite the fact that in amphibians spontaneous axonal regeneration of the optic nerve occurs, we show here that the molecule appears concomitantly with myelination during metamorphosis and is present in the adult optic nerve of the salamander Pleurodeles waltl by immunoblots and immunohistochemistry. In vitro, adult retinal ganglion cell axons were not able to grow from retinal explants on a tenascin-R substrate or to cross a sharp substrate border of tenascin-R in the presence of laminin, indicating that tenascin-R inhibits regrowth of retinal ganglion cell axons. After an optic nerve crush, immunoreactivity for tenascin-R was reduced to undetectable levels within 8 d. Immunoreactivity for the myelin-associated glycoprotein (MAG) was also diminished by that time. Myelin was removed by phagocytosing cells at 8-14 d after the lesion, as demonstrated by electron microscopy. Tenascin-R immunoreactivity was again detectable at 6 months after the lesion, correlated with remyelination as indicated by MAG immunohistochemistry. Regenerating axons began to repopulate the distal lesioned nerve at 9 d after a crush and grew in close contact with putative astrocytic processes in the periphery of the nerve, close to the pia, as demonstrated by anterograde tracing. Thus, the onset of axonal regrowth over the lesion site was correlated with the removal of inhibitory molecules in the optic nerve, which may be necessary for successful axonal regeneration in the CNS of amphibians.
Figures







References
-
- Bartsch U, Pesheva P, Raff M, Schachner M. Expression of janusin (J1–160/180) in the retina and optic nerve of the developing and adult mouse. Glia. 1993;9:57–69. - PubMed
-
- Bartsch U, Bandtlow CE, Schnell L, Bartsch S, Spillmann AA, Rubin BP, Hillenbrand R, Montag D, Schwab ME, Schachner M. Lack of evidence that myelin-associated glycoprotein is a major inhibitor of axonal regeneration in the CNS. Neuron. 1995;15:1375–1381. - PubMed
-
- Bates CA, Meyer RL. The neurite-promoting effect of laminin is mediated by different mechanisms in embryonic and adult regenerating mouse optic axons in vitro. Dev Biol. 1997;181:91–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
