Rapid resetting of the mammalian circadian clock
- PMID: 9880602
- PMCID: PMC6782190
- DOI: 10.1523/JNEUROSCI.19-02-00828.1999
Rapid resetting of the mammalian circadian clock
Abstract
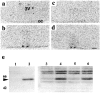
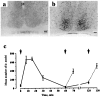
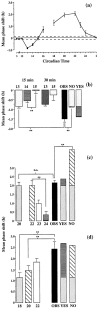
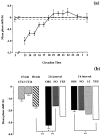
The suprachiasmatic nuclei (SCN) contain the principal circadian clock governing overt daily rhythms of physiology and behavior. The endogenous circadian cycle is entrained to the light/dark via direct glutamatergic retinal afferents to the SCN. To understand the molecular basis of entrainment, it is first necessary to define how rapidly the clock is reset by a light pulse. We used a two-pulse paradigm, in combination with cellular and behavioral analyses of SCN function, to explore the speed of resetting of the circadian oscillator in Syrian hamster and mouse. Analysis of c-fos induction and cAMP response element-binding protein phosphorylation in the retinorecipient SCN demonstrated that the SCN are able to resolve and respond to light pulses presented 1 or 2 hr apart. Analysis of the phase shifts of the circadian wheel-running activity rhythm of hamsters presented with single or double pulses demonstrated that resetting of the oscillator occurred within 2 hr. This was the case for both delaying and advancing phase shifts. Examination of delaying shifts in the mouse showed resetting within 2 hr and in addition showed that resetting is not completed within 1 hr of a light pulse. These results establish the temporal window within which to define the primary molecular mechanisms of circadian resetting in the mammal.
Figures


References
-
- Abe H, Rusak B. Physiological mechanisms regulating photic induction of Fos-like protein in hamster suprachiasmatic nucleus. Neurosci Biobehav Rev. 1994;18:531–536. - PubMed
-
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. - PubMed
-
- Aschoff J. Biological rhythms. Handbook of behavioral neurobiology, Vol 4 (Aschoff J, ed). Plenum; New York: 1981.
-
- Balsabore A, Damiola F, Schibler U. A serum-shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. - PubMed
-
- Binkley S, Mosher K. Circadian rhythm resetting in sparrows: early response to doublet light pulses. J Biol Rhythms. 1987;2:1–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
