Analysis of synthesis, stability, phosphorylation, and interacting polypeptides of the 34-kilodalton product of open reading frame 6 of the early region 4 protein of human adenovirus type 5
- PMID: 9882328
- PMCID: PMC103947
- DOI: 10.1128/JVI.73.2.1245-1253.1999
Analysis of synthesis, stability, phosphorylation, and interacting polypeptides of the 34-kilodalton product of open reading frame 6 of the early region 4 protein of human adenovirus type 5
Abstract
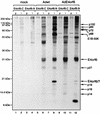
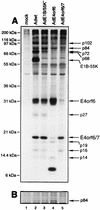
The 34-kDa early-region 4 open reading frame 6 (E4orf6) product of human adenovirus type 5 forms complexes with both the cellular tumor suppressor p53 and the viral E1B 55-kDa protein (E1B-55kDa). E4orf6 can inhibit p53 transactivation activity, as can E1B-55kDa, and in combination these viral proteins cause the rapid turnover of p53. In addition, E4orf6-55kDa complexes play a critical role at later times in the regulation of viral mRNA transport and shutoff of host cell protein synthesis. In the present study, we have further characterized some of the biological properties of E4orf6. Analysis of extracts from infected cells by Western blotting indicated that E4orf6, like E1A and E1B products, is present at high levels until very late times, suggesting that it is available to act throughout the infectious cycle. This pattern is similar to that of E4orf4 but differs markedly from that of another E4 product, E4orf6/7, which is present only transiently. Synthesis of E4orf6 is maximal at early stages but ceases completely with the onset of shutoff of host protein synthesis; however, it was found that unlike E4orf6/7, E4orf6 is very stable, thus allowing high levels to be maintained even at late times. E4orf6 was shown to be phosphorylated at low levels. Coimmunoprecipitation studies in cells lacking p53 indicated that E4orf6 interacts with a number of other proteins. Five of these were shown to be viral or virally induced proteins ranging in size from 102 to 27 kDa, including E1B-55kDa. One such species, of 72 kDa, was shown not to represent the E2 DNA-binding protein and thus remains to be identified. Another appeared to be the L4 100-kDa nonstructural adenovirus late product, but it appeared to be present nonspecifically and not as part of an E4orf6 complex. Apart from p53, three additional cellular proteins, of 84, 19, and 14 kDa were detected by using an adenovirus vector that expresses only E4orf6. The 19-kDa species and a 16-kDa cellular protein were also shown to interact with E4orf6/7. It is possible that complex formation with these viral and cellular proteins plays a role in one or more of the biological activities associated with E4orf6 and E4orf6/7.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

