Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins
- PMID: 9882340
- PMCID: PMC103959
- DOI: 10.1128/JVI.73.2.1350-1361.1999
Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins
Abstract
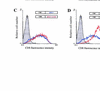
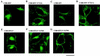
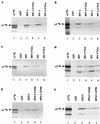
The cytoplasmic domains of the transmembrane (TM) envelope proteins (TM-CDs) of most retroviruses have a Tyr-based motif, YXXO, in their membrane-proximal regions. This signal is involved in the trafficking and endocytosis of membrane receptors via clathrin-associated AP-1 and AP-2 adaptor complexes. We have used CD8-TM-CD chimeras to investigate the role of the Tyr-based motif of human immunodeficiency virus type 1 (HIV-1), simian immunodeficiency virus (SIV), and human T-leukemia virus type 1 (HTLV-1) TM-CDs in the cell surface expression of the envelope glycoprotein. Flow cytometry and confocal microscopy studies showed that this motif is a major determinant of the cell surface expression of the CD8-HTLV chimera. The YXXO motif also plays a key role in subcellular distribution of the envelope of lentiviruses HIV-1 and SIV. However, these viruses, which encode TM proteins with a long cytoplasmic domain, have additional determinants distal to the YXXO motif that participate in regulating cell surface expression. We have also used the yeast two-hybrid system and in vitro binding assays to demonstrate that all three retroviral YXXO motifs interact with the micro1 and micro2 subunits of AP complexes and that the C-terminal regions of HIV-1 and SIV TM proteins interact with the beta2 adaptin subunit. The TM-CDs of HTLV-1, HIV-1, and SIV also interact with the whole AP complexes. These results clearly demonstrate that the cell surface expression of retroviral envelope glycoproteins is governed by interactions with adaptor complexes. The YXXO-based signal is the major determinant of this interaction for the HTLV-1 TM, which contains a short cytoplasmic domain, whereas the lentiviruses HIV-1 and SIV have additional determinants distal to this signal that are also involved.
Figures



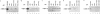

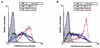
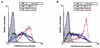
References
-
- Aiken C, Konner J, Landau N R, Lenburg M E, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1988;76:853–864. - PubMed
-
- Benichou S, Bomsel M, Bodeus M, Durand H, Doute M, Letourneur F, Camonis J, Benarous R. Physical interaction between the HIV-1 Nef protein and β-COP, an essential component for membrane traffic. J Biol Chem. 1994;269:30073–30076. - PubMed
-
- Benmerah A, Begue B, Dautry-Varsat A, Cerf-Bensussan N. The ear of α-adaptin interacts with the COOH-terminal domain of the Eps15 protein. J Biol Chem. 1996;271:12111–12116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

