Contribution of Ca2+-activated K+ channels and non-selective cation channels to membrane potential of pulmonary arterial smooth muscle cells of the rabbit
- PMID: 9882747
- PMCID: PMC2269107
- DOI: 10.1111/j.1469-7793.1999.747ad.x
Contribution of Ca2+-activated K+ channels and non-selective cation channels to membrane potential of pulmonary arterial smooth muscle cells of the rabbit
Abstract
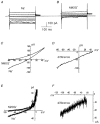
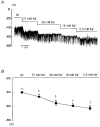
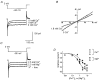
1. Using the perforated patch-clamp or whole-cell clamp technique, we investigated the contribution of Ca2+-activated K+ current (IK(Ca)) and non-selective cation currents (INSC) to the membrane potential in small pulmonary arterial smooth muscle cells of the rabbit. 2. The resting membrane potential (Vm) was -39.2 +/- 0.9 mV (n = 72). It did not stay at a constant level, but hyperpolarized irregularly, showing spontaneous transient hyperpolarizations (STHPs). The mean frequency and amplitude of the STHPs was 5.6 +/- 1. 1 Hz and -7.7 +/- 0.7 mV (n = 12), respectively. In the voltage-clamp mode, spontaneous transient outward currents (STOCs) were recorded with similar frequency and irregularity. 3. Intracellular application of BAPTA or extracellular application of TEA or charybdotoxin suppressed both the STHPs and STOCs. The depletion of intracellular Ca2+ stores by caffeine or ryanodine, and the removal of extracellular Ca2+ also abolished STHPs and STOCs. 4. Replacement of extracellular Na+ with NMDG+ caused hyperpolarization Vm of without affecting STHPs. Removal of extracellular Ca2+ induced a marked depolarization of Vm along with the disappearance of STHPs. 5. The ionic nature of the background inward current was identified. The permeability ratio of K+ : Cs+ : Na+ : Li+ was 1.7 : 1.3 : 1 : 0. 9, indicating that it is a non-selective cation current (INSC). The reversal potential of this current in control conditions was calculated to be -13.9 mV. The current was blocked by millimolar concentrations of extracellular Ca2+ and Mg2+. 6. From these results, it was concluded that (i) hyperpolarizing currents are mainly contributed by Ca2+-activated K+ (KCa) channels, and thus STOCs result in transient membrane hyperpolarization, and (ii) depolarizing currents are carried through NSC channels.
Figures

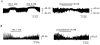


References
-
- Daut J, Standen NB, Nelson MT. The role of the membrane potential of endothelial and smooth muscle cells in the regulation of coronary blood flow. Journal of Cardiovascular Electrophysiology. 1994;5:154–181. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

