A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse
- PMID: 9884331
- PMCID: PMC407861
- DOI: 10.1172/JCI4431
A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse
Abstract
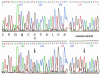
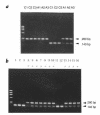
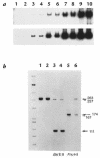
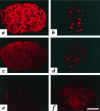
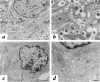
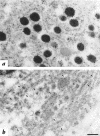
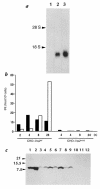
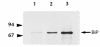
The mouse autosomal dominant mutation Mody develops hyperglycemia with notable pancreatic beta-cell dysfunction. This study demonstrates that one of the alleles of the gene for insulin 2 in Mody mice encodes a protein product that substitutes tyrosine for cysteine at the seventh amino acid of the A chain in its mature form. This mutation disrupts a disulfide bond between the A and B chains and can induce a drastic conformational change of this molecule. Although there was no gross defect in the transcription from the wild-type insulin 2 allele or two alleles of insulin 1, levels of proinsulin and insulin were profoundly diminished in the beta cells of Mody mice, suggesting that the number of wild-type (pro)insulin molecules was also decreased. Electron microscopy revealed a dramatic reduction of secretory granules and a remarkably enlarged lumen of the endoplasmic reticulum. Little proinsulin was processed to insulin, but high molecular weight forms of proinsulin existed with concomitant overexpression of BiP, a molecular chaperone in the endoplasmic reticulum. Furthermore, mutant proinsulin expressed in Chinese hamster ovary cells was inefficiently secreted, and its intracellular fraction formed complexes with BiP and was eventually degraded. These findings indicate that mutant proinsulin was trapped and accumulated in the endoplasmic reticulum, which could induce beta-cell dysfunction and account for the dominant phenotype of this mutation.
Figures


References
-
- King H, Rewers M. WHO Ad Hoc Diabetes Reporting Group. Global estimates for prevalence of diabetes mellitus and impaired glucose tolerance in adults. Diabetes Care. 1993;16:157–177. - PubMed
-
- Fajans SS. Scope and heterogeneous nature of MODY. Diabetes Care. 1990;13:49–64. - PubMed
-
- Froguel P, Vaxillaire M, Velho G. Genetic and metabolic heterogeneity of maturity-onset diabetes of the young. Diabetes Rev. 1997;5:123–130.
-
- Leiter EH, Herberg L. The polygenetics of diabesity in mice. Diabetes Rev. 1997;5:131–148.
-
- Yoshioka M, Kayo T, Ikeda T, Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

