Decreased angiogenesis and arthritic disease in rabbits treated with an alphavbeta3 antagonist
- PMID: 9884333
- PMCID: PMC407856
- DOI: 10.1172/JCI3756
Decreased angiogenesis and arthritic disease in rabbits treated with an alphavbeta3 antagonist
Abstract
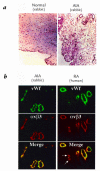
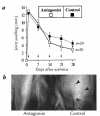
Rheumatoid arthritis (RA) is an inflammatory disease associated with intense angiogenesis and vascular expression of integrin alphavbeta3. Intra-articular administration of a cyclic peptide antagonist of integrin alphavbeta3 to rabbits with antigen-induced arthritis early in disease resulted in inhibition of synovial angiogenesis and reduced synovial cell infiltrate, pannus formation, and cartilage erosions. These effects were not associated with lymphopenia or impairment of leukocyte function. Furthermore, when administered in chronic, preexisting disease, the alphavbeta3 antagonist effectively diminished arthritis severity and was associated with a quantitative increase in apoptosis of the angiogenic blood vessels. Therefore, angiogenesis appears to be a central factor in the initiation and persistence of arthritic disease, and antagonists of integrin alphavbeta3 may represent a novel therapeutic strategy for RA.
Figures
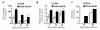




Comment in
-
Starving the synovium: angiogenesis and inflammation in rheumatoid arthritis.J Clin Invest. 1999 Jan;103(1):3-4. doi: 10.1172/JCI5929. J Clin Invest. 1999. PMID: 9884327 Free PMC article. No abstract available.
References
-
- Zvaifler NJ, Firestein GS. Pannus and pannocytes: alternative models of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1994;37:783–789. - PubMed
-
- Kimball ES, Gross JL. Angiogenesis in pannus formation. Agents Actions. 1991;34:329–331. - PubMed
-
- Qu Z, et al. Expression of basic fibroblast growth factor in synovial tissue from patients with rheumatoid arthritis and degenerative joint disease. Lab Invest. 1995;73:339–346. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

