Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency
- PMID: 9884342
- PMCID: PMC407858
- DOI: 10.1172/JCI4165
Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency
Abstract
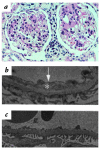
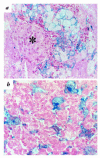
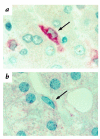
The first known human case of heme oxygenase-1 (HO-1) deficiency is presented in this report. The patient is a six-year-old boy with severe growth retardation. He has been suffering from persistent hemolytic anemia characterized by marked erythrocyte fragmentation and intravascular hemolysis, with paradoxical increase of serum haptoglobin and low bilirubin. An abnormal coagulation/fibrinolysis system, associated with elevated thrombomodulin and von Willebrand factor, indicated the presence of severe, persistent endothelial damage. Electron microscopy of renal glomeruli revealed detachment of endothelium, with subendothelial deposition of an unidentified material. Iron deposition was noted in renal and hepatic tissue. Immunohistochemistry of hepatic tissue and immunoblotting of a cadmium-stimulated Epstein-Barr virus-transformed lymphoblastoid cell line (LCL) revealed complete absence of HO-1 production. An LCL derived from the patient was extremely sensitive to hemin-induced cell injury. Sequence analysis of the patient's HO-1 gene revealed complete loss of exon-2 of the maternal allele and a two-nucleotide deletion within exon3 of the paternal allele. Growth retardation, anemia, iron deposition, and vulnerability to stressful injury are all characteristics observed in recently described HO-1 targeted mice. This study presents not only the first human case of HO-1 deficiency but may also provide clues to the key roles played by this important enzyme in vivo.
Figures





References
-
- Maines MD. Heme oxygenase: function, multiplicity, regulatory mechanism, and clinical applications. FASEB J. 1988;2:2557–2568. - PubMed
-
- Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. - PubMed
-
- Okinaga S, et al. Regulation of human heme oxygenase-1 gene expression under thermal stress. Blood. 1996;87:5074–5084. - PubMed
-
- Willis D, Moore AR, Frederick R, Willoughby DA. Heme oxygenase: a novel target for the modulation of the inflammatory response. Nat Med. 1996;2:87–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

