Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation
- PMID: 9885252
- PMCID: PMC2148125
- DOI: 10.1083/jcb.144.1.161
Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation
Abstract
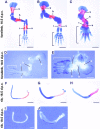
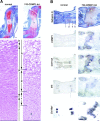
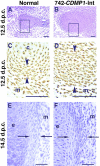
Cartilage provides the template for endochondral ossification and is crucial for determining the length and width of the skeleton. Transgenic mice with targeted expression of recombinant cartilage-derived morphogenetic protein-1 (CDMP-1), a member of the bone morphogenetic protein family, were created to investigate the role of CDMP-1 in skeletal formation. The mice exhibited chondrodysplasia with expanded cartilage, which consists of the enlarged hypertrophic zone and the reduced proliferating chondrocyte zone. Histologically, CDMP-1 increased the number of chondroprogenitor cells and accelerated chondrocyte differentiation to hypertrophy. Expression of CDMP-1 in the notochord inhibited vertebral body formation by blocking migration of sclerotome cells to the notochord. These results indicate that CDMP-1 antagonizes the ventralization signals from the notochord. Our study suggests a molecular mechanism by which CDMP-1 regulates the formation, growth, and differentiation of the skeletal elements.
Figures






References
-
- Chang SC, Hoang B, Thomas JT, Vukicevic S, Luyten FP, Ryba NJ, Kozak CA, Reddi AH, Moos M., Jr Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-β superfamily predominantly expressed in long bones during human embryonic development. J Biol Chem. 1994;269:28227–28234. - PubMed
-
- Christ B, Wilting J. From somites to vertebral column. Anat Anz. 1992;174:23–32. - PubMed
-
- Crocker J, Nar P. Nuclear organizer regions in lymphomas. J Pathol. 1987;151:111–118. - PubMed
-
- Deutsch U, Dressler GR, Gruss P. Pax 1, a member of a paired box homologous murine gene family, is expressed in segmented structures during development. Cell. 1988;53:617–625. - PubMed
-
- Dewulf N, Verschueren K, Lonnoy O, Morén A, Grimsby S, Vande K, Spiegle, Miyazono K, Huylebroeck D, Ten P, Dijke Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

