Isolation and functional characterization of two distinct sexual-stage-specific promoters of the human malaria parasite Plasmodium falciparum
- PMID: 9891033
- PMCID: PMC116028
- DOI: 10.1128/MCB.19.2.967
Isolation and functional characterization of two distinct sexual-stage-specific promoters of the human malaria parasite Plasmodium falciparum
Abstract
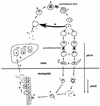
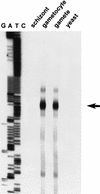
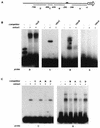
Transmission of malaria depends on the successful development of the sexual stages of the parasite within the midgut of the mosquito vector. The differentiation process leading to the production of the sexual stages is delineated by several developmental switches. Arresting the progression through this sexual differentiation pathway would effectively block the spread of the disease. The successful development of such transmission-blocking agents is hampered by the lack of a detailed understanding of the program of gene expression that governs sexual differentiation of the parasite. Here we describe the isolation and functional characterization of the Plasmodium falciparum pfs16 and pfs25 promoters, whose activation marks the developmental switches executed during the sexual differentiation process. We have studied the differential activation of the pfs16 and pfs25 promoters during intraerythrocytic development by transfection of P. falciparum and during gametogenesis and early sporogonic development by transfection of the related malarial parasite P. gallinaceum. Our data indicate that the promoter of the pfs16 gene is activated at the onset of gametocytogenesis, while the activity of the pfs25 promoter is induced following the transition to the mosquito vector. Both promoters have unusual DNA compositions and are extremely A/T rich. We have identified the regions in the pfs16 and pfs25 promoters that are essential for high transcriptional activity. Furthermore, we have identified a DNA-binding protein, termed PAF-1, which activates pfs25 transcription in the mosquito midgut. The data presented here shed the first light on the details of processes of gene regulation in the important human pathogen P. falciparum.
Figures









Similar articles
-
Developmentally regulated expression of pfs16, a marker for sexual differentiation of the human malaria parasite Plasmodium falciparum.Mol Biochem Parasitol. 1997 Nov;89(2):235-44. doi: 10.1016/s0166-6851(97)00123-0. Mol Biochem Parasitol. 1997. PMID: 9364968
-
Sexual forms obtained in a continuous in vitro cultured Colombian strain of Plasmodium falciparum (FCB2).Malar J. 2020 Feb 3;19(1):57. doi: 10.1186/s12936-020-3142-y. Malar J. 2020. PMID: 32014000 Free PMC article.
-
[Biology of man-mosquito Plasmodium transmission].Bull Soc Pathol Exot. 2003 Mar;96(1):6-20. Bull Soc Pathol Exot. 2003. PMID: 12784587 Review. French.
-
Quantification of Plasmodium falciparum gametocytes in differential stages of development by quantitative nucleic acid sequence-based amplification.Mol Biochem Parasitol. 2004 Sep;137(1):35-41. doi: 10.1016/j.molbiopara.2004.03.018. Mol Biochem Parasitol. 2004. PMID: 15279949
-
Plasmodium falciparum goes bananas for sex.Mol Biochem Parasitol. 2021 Jul;244:111385. doi: 10.1016/j.molbiopara.2021.111385. Epub 2021 May 29. Mol Biochem Parasitol. 2021. PMID: 34062177 Review.
Cited by
-
Integrative analysis of intraerythrocytic differentially expressed transcripts yields novel insights into the biology of Plasmodium falciparum.Malar J. 2003 Nov 14;2(1):38. doi: 10.1186/1475-2875-2-38. Malar J. 2003. PMID: 14617379 Free PMC article.
-
Serial analysis of gene expression in Plasmodium falciparum reveals the global expression profile of erythrocytic stages and the presence of anti-sense transcripts in the malarial parasite.Mol Biol Cell. 2001 Oct;12(10):3114-25. doi: 10.1091/mbc.12.10.3114. Mol Biol Cell. 2001. PMID: 11598196 Free PMC article.
-
Diverse expression patterns of subgroups of the rif multigene family during Plasmodium falciparum gametocytogenesis.PLoS One. 2008;3(11):e3779. doi: 10.1371/journal.pone.0003779. Epub 2008 Nov 20. PLoS One. 2008. PMID: 19020666 Free PMC article.
-
A semi-automated luminescence based standard membrane feeding assay identifies novel small molecules that inhibit transmission of malaria parasites by mosquitoes.Sci Rep. 2015 Dec 21;5:18704. doi: 10.1038/srep18704. Sci Rep. 2015. PMID: 26687564 Free PMC article.
-
In vivo and in vitro characterization of a Plasmodium liver stage-specific promoter.PLoS One. 2015 Apr 15;10(4):e0123473. doi: 10.1371/journal.pone.0123473. eCollection 2015. PLoS One. 2015. PMID: 25874388 Free PMC article.
References
-
- Alano P, Carter R. Sexual differentiation in malaria parasites. Annu Rev Microbiol. 1990;44:429–449. - PubMed
-
- Baker D A, Daramola O, McCrossan M V, Harmer J, Targett G A T. Subcellular localization of Pfs16, a Plasmodium falciparum gametocyte antigen. Parasitology. 1994;108:129–137. - PubMed
-
- Billker O, Lindo V, Panico M, Etienne A E, Paxton T, Dell A, Rogers M, Sinden R E, Morris H R. Identification of xanthurenic acid as the putative inducer of malaria development in the mosquito. Nature. 1998;392:289–292. - PubMed
-
- Bohne W, Wirsing A, Gross U. Bradyzoite-specific gene expression in Toxoplasma gondii requires minimal genomic elements. Mol Biochem Parasitol. 1997;85:89–98. - PubMed
-
- Bruce M C, Alano P, Carter R. Commitment of the malaria parasite Plasmodium falciparum to sexual and asexual development. Parasitology. 1990;100:191–200. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
