An SH2 domain-containing 5' inositolphosphatase inhibits insulin-induced GLUT4 translocation and growth factor-induced actin filament rearrangement
- PMID: 9891043
- PMCID: PMC116038
- DOI: 10.1128/MCB.19.2.1081
An SH2 domain-containing 5' inositolphosphatase inhibits insulin-induced GLUT4 translocation and growth factor-induced actin filament rearrangement
Abstract
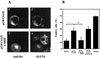
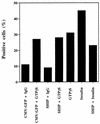
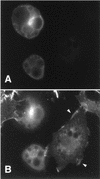
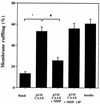
Tyrosine kinase receptors lead to rapid activation of phosphatidylinositol 3-kinase (PI3 kinase) and the subsequent formation of phosphatidylinositides (PtdIns) 3,4-P2 and PtdIns 3,4, 5-P3, which are thought to be involved in signaling for glucose transporter GLUT4 translocation, cytoskeletal rearrangement, and DNA synthesis. However, the specific role of each of these PtdIns in insulin and growth factor signaling is still mainly unknown. Therefore, we assessed, in the current study, the effect of SH2-containing inositol phosphatase (SHIP) expression on these biological effects. SHIP is a 5' phosphatase that decreases the intracellular levels of PtdIns 3,4,5-P3. Expression of SHIP after nuclear microinjection in 3T3-L1 adipocytes inhibited insulin-induced GLUT4 translocation by 100 +/- 21% (mean +/- the standard error) at submaximal (3 ng/ml) and 64 +/- 5% at maximal (10 ng/ml) insulin concentrations (P < 0.05 and P < 0.001, respectively). A catalytically inactive mutant of SHIP had no effect on insulin-induced GLUT4 translocation. Furthermore, SHIP also abolished GLUT4 translocation induced by a membrane-targeted catalytic subunit of PI3 kinase. In addition, insulin-, insulin-like growth factor I (IGF-I)-, and platelet-derived growth factor-induced cytoskeletal rearrangement, i.e., membrane ruffling, was significantly inhibited (78 +/- 10, 64 +/- 3, and 62 +/- 5%, respectively; P < 0.05 for all) in 3T3-L1 adipocytes. In a rat fibroblast cell line overexpressing the human insulin receptor (HIRc-B), SHIP inhibited membrane ruffling induced by insulin and IGF-I by 76 +/- 3% (P < 0.001) and 68 +/- 5% (P < 0.005), respectively. However, growth factor-induced stress fiber breakdown was not affected by SHIP expression. Finally, SHIP decreased significantly growth factor-induced mitogen-activated protein kinase activation and DNA synthesis. Expression of the catalytically inactive mutant had no effect on these cellular responses. In summary, our results show that expression of SHIP inhibits insulin-induced GLUT4 translocation, growth factor-induced membrane ruffling, and DNA synthesis, indicating that PtdIns 3,4,5-P3 is the key phospholipid product mediating these biological actions.
Figures






Similar articles
-
Overexpression of SH2-containing inositol phosphatase 2 results in negative regulation of insulin-induced metabolic actions in 3T3-L1 adipocytes via its 5'-phosphatase catalytic activity.Mol Cell Biol. 2001 Mar;21(5):1633-46. doi: 10.1128/MCB.21.5.1633-1646.2001. Mol Cell Biol. 2001. PMID: 11238900 Free PMC article.
-
Effects of general receptor for phosphoinositides 1 on insulin and insulin-like growth factor I-induced cytoskeletal rearrangement, glucose transporter-4 translocation, and deoxyribonucleic acid synthesis.Endocrinology. 1998 Dec;139(12):4984-90. doi: 10.1210/endo.139.12.6351. Endocrinology. 1998. PMID: 9832437
-
Dual role of SRC homology domain 2-containing inositol phosphatase 2 in the regulation of platelet-derived growth factor and insulin-like growth factor I signaling in rat vascular smooth muscle cells.Endocrinology. 2003 Sep;144(9):4204-14. doi: 10.1210/en.2003-0190. Endocrinology. 2003. PMID: 12933696
-
Role of the Src homology 2 (SH2) domain and C-terminus tyrosine phosphorylation sites of SH2-containing inositol phosphatase (SHIP) in the regulation of insulin-induced mitogenesis.Endocrinology. 1999 Oct;140(10):4585-94. doi: 10.1210/endo.140.10.7028. Endocrinology. 1999. PMID: 10499514
-
Phosphatidylinositol 3-phosphate [PtdIns3P] is generated at the plasma membrane by an inositol polyphosphate 5-phosphatase: endogenous PtdIns3P can promote GLUT4 translocation to the plasma membrane.Mol Cell Biol. 2006 Aug;26(16):6065-81. doi: 10.1128/MCB.00203-06. Mol Cell Biol. 2006. PMID: 16880518 Free PMC article.
Cited by
-
Pathogenicity of Salmonella: SopE-mediated membrane ruffling is independent of inositol phosphate signals.FEBS Lett. 2006 Mar 20;580(7):1709-15. doi: 10.1016/j.febslet.2006.02.019. Epub 2006 Feb 17. FEBS Lett. 2006. PMID: 16500648 Free PMC article.
-
Visualization of negative signaling in B cells by quantitative confocal microscopy.Mol Cell Biol. 2001 Dec;21(24):8615-25. doi: 10.1128/MCB.21.24.8615-8625.2001. Mol Cell Biol. 2001. PMID: 11713294 Free PMC article.
-
Chronic endothelin-1 treatment leads to heterologous desensitization of insulin signaling in 3T3-L1 adipocytes.J Clin Invest. 2001 May;107(9):1193-202. doi: 10.1172/JCI11753. J Clin Invest. 2001. PMID: 11342583 Free PMC article.
-
FOXO1 transrepresses peroxisome proliferator-activated receptor gamma transactivation, coordinating an insulin-induced feed-forward response in adipocytes.J Biol Chem. 2009 May 1;284(18):12188-97. doi: 10.1074/jbc.M808915200. Epub 2009 Feb 26. J Biol Chem. 2009. PMID: 19246449 Free PMC article.
-
Cloning, characterization, and expression of a novel Zn2+-binding FYVE finger-containing phosphoinositide kinase in insulin-sensitive cells.Mol Cell Biol. 1999 Jan;19(1):623-34. doi: 10.1128/MCB.19.1.623. Mol Cell Biol. 1999. PMID: 9858586 Free PMC article.
References
-
- Alessi D R, Deak M, Casamayor A, Caudwell F B, Morrice N, Norman D G, Gaffney P, Reese C B, MacDougall C N, Harbison D, Ashworth A, Bownes M. 3-Phosphoinositide-dependent protein kinase-1 (PDK-1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr Biol. 1997;7:776–789. - PubMed
-
- Alessi D R, James S R, Downes C P, Holmes A B, Gaffney P R J, Reese C B, Cohen P. Characterization of a 3-Phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. - PubMed
-
- Auger K, Serunian L A, Soltoff S P, Libby P, Cantley L C. PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell. 1989;57:167–175. - PubMed
-
- Burgering B M T, Coffer P J. Protein kinase B (c-Akt) in phosphatidylinositol-3-OH kinase signal transduction. Nature. 1995;376:599–602. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
