Extracellular signal-regulated kinase 7 (ERK7), a novel ERK with a C-terminal domain that regulates its activity, its cellular localization, and cell growth
- PMID: 9891064
- PMCID: PMC116059
- DOI: 10.1128/MCB.19.2.1301
Extracellular signal-regulated kinase 7 (ERK7), a novel ERK with a C-terminal domain that regulates its activity, its cellular localization, and cell growth
Abstract
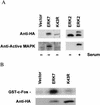
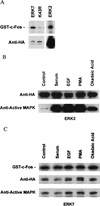
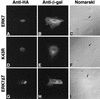
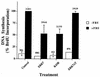
Mitogen-activated protein (MAP) kinases play distinct roles in a variety of cellular signaling pathways and are regulated through multiple mechanisms. In this study, a novel 61-kDa member of the MAP kinase family, termed extracellular signal-regulated kinase 7 (ERK7), has been cloned and characterized. Although it has the signature TEY activation motif of ERK1 and ERK2, ERK7 is not activated by extracellular stimuli that typically activate ERK1 and ERK2 or by common activators of c-Jun N-terminal kinase (JNK) and p38 kinase. Instead, ERK7 has appreciable constitutive activity in serum-starved cells that is dependent on the presence of its C-terminal domain. Interestingly, the C-terminal tail, not the kinase domain, of ERK7 regulates its nuclear localization and inhibition of growth. Taken together, these results elucidate a novel type of MAP kinase whereby interactions via its C-terminal tail, rather than extracellular signal-mediated activation cascades, regulate its activity, localization, and function.
Figures





References
-
- Abe J, Kusuhara M, Ulevitch R J, Berk B C, Lee J-D. Big mitogen-activated protein kinase 1 (BMK1) is a redox-sensitive kinase. J Biol Chem. 1996;271:16586–16590. - PubMed
-
- Abe J, Takahashi M, Ishida M, Lee J-D, Berk B C. c-Src is required for oxidative stress-mediated activation of big mitogen-activated protein kinase 1. J Biol Chem. 1997;272:20389–20394. - PubMed
-
- Abe M K, Chao T S, Solway J, Rosner M R, Hershenson M B. Hydrogen peroxide stimulates mitogen-activated protein kinase in bovine tracheal myocytes: implications for human airway disease. Am J Respir Cell Mol Biol. 1994;11:577–585. - PubMed
-
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Anderson N G, Maller J L, Tonks N K, Sturgill T W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990;343:651–653. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
