Essential role of the dynamin pleckstrin homology domain in receptor-mediated endocytosis
- PMID: 9891074
- PMCID: PMC116069
- DOI: 10.1128/MCB.19.2.1410
Essential role of the dynamin pleckstrin homology domain in receptor-mediated endocytosis
Abstract
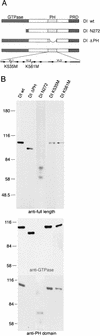
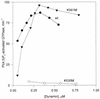
Pleckstrin homology (PH) domains are found in numerous membrane-associated proteins and have been implicated in the mediation of protein-protein and protein-phospholipid interactions. Dynamin, a GTPase required for clathrin-dependent endocytosis, contains a PH domain which binds to phosphoinositides and participates in the interaction between dynamin and the betagamma subunits of heterotrimeric G proteins. The PH domain is essential for expression of phosphoinositide-stimulated GTPase activity of dynamin in vitro, but its involvement in the endocytic process is unknown. We expressed a series of dynamin PH domain mutants in cultured cells and determined their effect on transferrin uptake by those cells. Endocytosis is blocked in cells expressing a PH domain deletion mutant and a point mutant that fails to interact with phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2]. In contrast, expression of a point mutant with unimpaired PI(4,5)P2 interaction has no effect on transferrin uptake. These results demonstrate the significance of the PH domain for dynamin function and suggest that its role may be to mediate interactions between dynamin and phosphoinositides.
Figures


References
-
- Barylko B, Binns D, Lin K M, Atkinson M A L, Jameson D M, Yin H L, Albanesi J P. Synergistic activation of dynamin GTPase by GRB2 and phosphoinositides. J Biol Chem. 1998;273:3791–3797. - PubMed
-
- Bauerfeind R, Takei K, De Camilli P. Amphiphysin I is associated with coated endocytic intermediates and undergoes stimulation-dependent dephosphorylation in nerve terminals. J Biol Chem. 1997;272:30984–30992. - PubMed
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
