rlk/TXK encodes two forms of a novel cysteine string tyrosine kinase activated by Src family kinases
- PMID: 9891083
- PMCID: PMC116078
- DOI: 10.1128/MCB.19.2.1498
rlk/TXK encodes two forms of a novel cysteine string tyrosine kinase activated by Src family kinases
Abstract
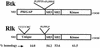
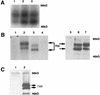
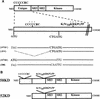
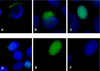
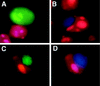
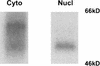
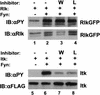
Rlk/Txk is a member of the BTK/Tec family of tyrosine kinases and is primarily expressed in T lymphocytes. Unlike other members of this kinase family, Rlk lacks a pleckstrin homology (PH) domain near the amino terminus and instead contains a distinctive cysteine string motif. We demonstrate here that Rlk protein consists of two isoforms that arise by alternative initiation of translation from the same cDNA. The shorter, internally initiated protein species lacks the cysteine string motif and is located in the nucleus when expressed in the absence of the larger form. In contrast, the larger form is cytoplasmic. We show that the larger form is palmitoylated and that mutation of its cysteine string motif both abolishes palmitoylation and allows the protein to migrate to the nucleus. The cysteine string, therefore, is a critical determinant of both fatty acid modification and protein localization for the larger isoform of Rlk, suggesting that Rlk regulation is distinct from the other Btk family kinases. We further show that Rlk is phosphorylated and changes localization in response to T-cell-receptor (TCR) activation and, like the other Btk family kinases, can be phosphorylated and activated by Src family kinases. However, unlike the other Btk family members, Rlk is activated independently of the activity of phosphatidylinositol 3-kinase, consistent with its lack of a PH domain. Thus, Rlk has two distinct isoforms, each of which may have unique properties in signaling downstream from the TCR.
Figures





References
-
- Braun J E, Scheller R H. Cysteine string protein, a DnaJ family member, is present on diverse secretory vesicles. Neuropharmacology. 1995;34:1361–1369. - PubMed
-
- Cheng, G., M. Chamorro, and P. L. Schwartzberg. Unpublished observations.
-
- Debnath, J. Unpublished observations.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
