Biochemical nature and cellular distribution of the paired immunoglobulin-like receptors, PIR-A and PIR-B
- PMID: 9892613
- PMCID: PMC2192985
- DOI: 10.1084/jem.189.2.309
Biochemical nature and cellular distribution of the paired immunoglobulin-like receptors, PIR-A and PIR-B
Abstract
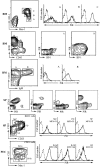
PIR-A and PIR-B, paired immunoglobulin-like receptors encoded, respectively, by multiple Pira genes and a single Pirb gene in mice, are relatives of the human natural killer (NK) and Fc receptors. Monoclonal and polyclonal antibodies produced against a recombinant PIR protein identified cell surface glycoproteins of approximately 85 and approximately 120 kD on B cells, granulocytes, and macrophages. A disulfide-linked homodimer associated with the cell surface PIR molecules was identified as the Fc receptor common gamma (FcRgammac) chain. Whereas PIR-B fibroblast transfectants expressed cell surface molecules of approximately 120 kD, PIR-A transfectants expressed the approximately 85-kD molecules exclusively intracellularly; PIR-A and FcRgammac cotransfectants expressed the PIR-A/ FcRgammac complex on their cell surface. Correspondingly, PIR-B was normally expressed on the cell surface of splenocytes from FcRgammac-/- mice whereas PIR-A was not. Cell surface levels of PIR molecules on myeloid and B lineage cells increased with cellular differentiation and activation. Dendritic cells, monocytes/macrophages, and mast cells expressed the PIR molecules in varying levels, but T cells and NK cells did not. These experiments define the coordinate cellular expression of PIR-B, an inhibitory receptor, and PIR-A, an activating receptor; demonstrate the requirement of FcRgammac chain association for cell surface PIR-A expression; and suggest that the level of FcRgammac chain expression could differentially affect the PIR-A/PIR-B equilibrium in different cell lineages.
Figures

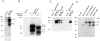
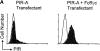
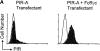


References
-
- Hayami K, Fukuta D, Nishikawa Y, Yamashita Y, Inui M, Ohyama Y, Hikida M, Ohmori H, Takai T. Molecular cloning of a novel murine cell-surface glycoprotein homologous to killer cell inhibitory receptors. J Biol Chem. 1997;272:7320–7327. - PubMed
-
- Colonna M, Samaridis J. Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells. Science. 1995;268:405–408. - PubMed
-
- Wagtmann N, Biassoni R, Cantoni C, Verdiani S, Malnati MS, Vitale M, Bottino C, Moretta L, Moretta A, Long EO. Molecular clones of the p58 NK cell receptor reveal immunoglobulin-related molecules with diversity in both the extra- and intracellular domains. Immunity. 1995;2:439–449. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

