Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis
- PMID: 9892616
- PMCID: PMC2192995
- DOI: 10.1084/jem.189.2.341
Targeted disruption of migration inhibitory factor gene reveals its critical role in sepsis
Abstract
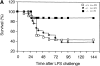
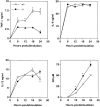
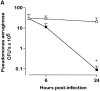
To study the biologic role of migration inhibitory factor (MIF), a pleiotropic cytokine, we generated a mouse strain lacking MIF by gene targeting in embryonic stem cells. Analysis of the role of MIF during sepsis showed that MIF-/- mice were resistant to the lethal effects of high dose bacterial lipopolysaccharide (LPS), or Staphylococcus aureus enterotoxin B (SEB) with D-galactosamine and had lower plasma levels of tumor necrosis factor alpha (TNF-alpha) than did wild-type mice, but normal levels of interleukin (IL)-6 and IL-10. When stimulated with LPS and interferon gamma, macrophages from MIF-/- mice showed diminished production of TNF-alpha, normal IL-6 and IL-12, and increased production of nitric oxide. MIF-/- animals cleared gram-negative bacteria Pseudomonas aeruginosa instilled into the trachea better than did wild-type mice and had diminished neutrophil accumulation in their bronchoalveolar fluid compared to the wild-type mice. Thioglycollate elicited peritoneal exudates in uninfected MIF-/- mice, but showed normal neutrophil accumulation. Finally, the findings of enhanced resistance to P. aeruginosa and resistance to endotoxin-induced lethal shock suggest that the counteraction or neutralization of MIF may serve as an adjunct therapy in sepsis.
Figures






References
-
- David, J.R., M. Bozza, and C. Carini. 1996. Macrophage migration inhibitory factor. In Human Cytokines. Handbook for Basic and Clinical Research, Vol. 2. B.B. Aggarwal and J.U. Gutterman, editors. Blackwell Science, Cambridge, MA. 222–256.
-
- Bucala R. MIF rediscovered: cytokine, pituitary hormone, and glucocorticoid-induced regulator of the immune response. FASEB J. 1996;10:1607–1613. - PubMed
-
- Bernhagen, J., T. Calandra, R.A. Mitchell, S.B. Martin, K.J. Tracey, W. Voelter, K.R. Manogue, A. Cerami, and R. Bucala. 1993. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 365:756–759. (See published erratum 378:419.) - PubMed
-
- Calandra T, Bernhagen J, Metz CN, Spiegel LA, Bacher M, Donnelly T, Cerami A, Bucala R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature. 1995;377:68–71. - PubMed
-
- Mikulowska A, Metz CN, Bucala R, Holmdahl R. Macrophage migration inhibitory factor is involved in the pathogenesis of collagen type II-induced arthritis in mice. J Immunol. 1997;158:5514–5517. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

