Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization
- PMID: 9892619
- PMCID: PMC2192989
- DOI: 10.1084/jem.189.2.371
Fcgamma receptor-mediated induction of dendritic cell maturation and major histocompatibility complex class I-restricted antigen presentation after immune complex internalization
Abstract
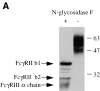
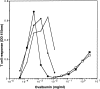
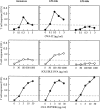
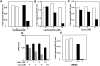
Dendritic cells (DCs) express several receptors for the Fc portion of immunoglobulin (Ig)G (FcgammaR), which mediate internalization of antigen-IgG complexes (immune complexes, ICs) and promote efficient major histocompatibility complex (MHC) class II-restricted antigen presentation. We now show that FcgammaRs have two additional specific attributes in murine DCs: the induction of DC maturation and the promotion of efficient MHC class I-restricted presentation of peptides from exogenous, IgG-complexed antigens. Both FcgammaR functions require the FcgammaR-associated gamma chain. FcgammaR-mediated MHC class I-restricted antigen presentation is extremely sensitive and specific to immature DCs. It requires proteasomal degradation and is dependent on functional peptide transporter associated with antigen processing, TAP1-TAP2. By promoting DC maturation and presentation on both MHC class I and II molecules, ICs should efficiently sensitize DCs for priming of both CD4(+) helper and CD8(+) cytotoxic T lymphocytes in vivo.
Figures



References
-
- Townsend A, Bodmer H. Antigen recognition by class I-restricted T lymphocytes. Annu Rev Immunol. 1989;7:601–624. - PubMed
-
- Huang AYC, Golumbeck P, Ahmadzadeh M, Jaffee A, Pardoll DM, Levitsky HI. Role of bone marrow-derived cells in presenting MHC class I-restricted tumor antigens. Science. 1994;264:961–965. - PubMed
-
- Albert ML, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. - PubMed
-
- Hart DNJ. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

