Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection
- PMID: 9892624
- PMCID: PMC2192982
- DOI: 10.1084/jem.189.2.423
Migration kinetics and final destination of type 1 and type 2 CD8 effector cells predict protection against pulmonary virus infection
Abstract
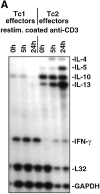
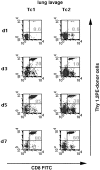
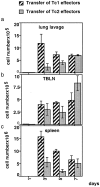
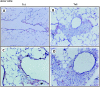
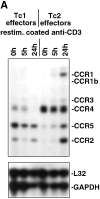
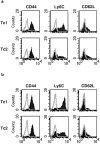
The requirements for CD8 T cells to provide protection against a localized virus infection in models of adoptive immunotherapy are not well defined. Here we investigated the protective value of defined in vitro-generated hemagglutinin (HA) peptide-specific primary CD8 T cell effectors from the clone 4 T cell receptor transgenic mice, secreting type 1 or type 2 cytokines, against pulmonary infection with whole influenza virus. Cytotoxic T lymphocytes producing type 1 and type 2 cytokine (Tc1 and Tc2) populations were equally cytolytic, but Tc1 effectors and not Tc2 effectors reduced the pulmonary virus titer early during infection. Host recovery mediated by Tc1 effectors was found to be independent of interferon gamma production. Tc2 effectors entered the lung with delayed kinetics as compared with Tc1 effectors, and after lung entry Tc2 effector cells did not localize near the infected airway epithelium as did Tc1 effectors but were found within clusters of inflammatory cells distant from the epithelium. We also show that the expression of several chemokine receptors was selectively regulated in the Tc1 and Tc2 subsets. Thus, the protective value of a CD8 cell population against pulmonary influenza virus infection is strongly correlated with its ability to exert its effector potential at the site of virus infection.
Figures






References
-
- Doherty PC, Topham DJ, Tripp RA, Cardin RD, Brooks JW, Stevenson PG. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. - PubMed
-
- Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev. 1997;159:95–103. - PubMed
-
- Yap KL, Ada GL, McKenzie IFC. Transfer of specific cytotoxic lymphocytes protects mice inoculated with influenza virus. Nature. 1978;238:238–239. - PubMed
-
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159:5197–5200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

