Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro
- PMID: 9892638
- PMCID: PMC15141
- DOI: 10.1073/pnas.96.2.361
Silicatein filaments and subunits from a marine sponge direct the polymerization of silica and silicones in vitro
Abstract
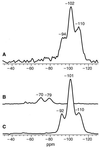
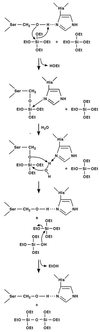
Nanoscale control of the polymerization of silicon and oxygen determines the structures and properties of a wide range of siloxane-based materials, including glasses, ceramics, mesoporous molecular sieves and catalysts, elastomers, resins, insulators, optical coatings, and photoluminescent polymers. In contrast to anthropogenic and geological syntheses of these materials that require extremes of temperature, pressure, or pH, living systems produce a remarkable diversity of nanostructured silicates at ambient temperatures and pressures and at near-neutral pH. We show here that the protein filaments and their constituent subunits comprising the axial cores of silica spicules in a marine sponge chemically and spatially direct the polymerization of silica and silicone polymer networks from the corresponding alkoxide substrates in vitro, under conditions in which such syntheses otherwise require either an acid or base catalyst. Homology of the principal protein to the well known enzyme cathepsin L points to a possible reaction mechanism that is supported by recent site-directed mutagenesis experiments. The catalytic activity of the "silicatein" (silica protein) molecule suggests new routes to the synthesis of silicon-based materials.
Figures

Similar articles
-
Silicatein: A Unique Silica-Synthesizing Catalytic Triad Hydrolase From Marine Sponge Skeletons and Its Multiple Applications.Methods Enzymol. 2018;605:429-455. doi: 10.1016/bs.mie.2018.02.025. Epub 2018 Apr 11. Methods Enzymol. 2018. PMID: 29909834
-
Efficient silica synthesis from tetra(glycerol)orthosilicate with cathepsin- and silicatein-like proteins.Sci Rep. 2018 Nov 13;8(1):16759. doi: 10.1038/s41598-018-34965-9. Sci Rep. 2018. PMID: 30425281 Free PMC article.
-
Silicatein alpha: cathepsin L-like protein in sponge biosilica.Proc Natl Acad Sci U S A. 1998 May 26;95(11):6234-8. doi: 10.1073/pnas.95.11.6234. Proc Natl Acad Sci U S A. 1998. PMID: 9600948 Free PMC article.
-
Molecular biology of demosponge axial filaments and their roles in biosilicification.Microsc Res Tech. 2003 Nov 1;62(4):356-67. doi: 10.1002/jemt.10401. Microsc Res Tech. 2003. PMID: 14534908 Review.
-
Silicateins, silicatein interactors and cellular interplay in sponge skeletogenesis: formation of glass fiber-like spicules.FEBS J. 2012 May;279(10):1721-36. doi: 10.1111/j.1742-4658.2012.08533.x. Epub 2012 Apr 17. FEBS J. 2012. PMID: 22340505 Review.
Cited by
-
Titanium mineralization in ferritin: a room temperature nonphotochemical preparation and biophysical characterization.J Biol Inorg Chem. 2013 Jan;18(1):145-52. doi: 10.1007/s00775-012-0959-z. Epub 2012 Nov 20. J Biol Inorg Chem. 2013. PMID: 23179270
-
Fractal intermediates in the self-assembly of silicatein filaments.Proc Natl Acad Sci U S A. 2005 Aug 16;102(33):11657-62. doi: 10.1073/pnas.0503968102. Epub 2005 Aug 9. Proc Natl Acad Sci U S A. 2005. PMID: 16091468 Free PMC article.
-
Inorganic Polymeric Materials for Injured Tissue Repair: Biocatalytic Formation and Exploitation.Biomedicines. 2022 Mar 11;10(3):658. doi: 10.3390/biomedicines10030658. Biomedicines. 2022. PMID: 35327460 Free PMC article. Review.
-
Distribution of microfossils within polymetallic nodules: biogenic clusters within manganese layers.Mar Biotechnol (NY). 2012 Feb;14(1):96-105. doi: 10.1007/s10126-011-9393-4. Epub 2011 May 31. Mar Biotechnol (NY). 2012. PMID: 21626367
-
Biomimetic model systems for investigating the amorphous precursor pathway and its role in biomineralization.Chem Rev. 2008 Nov;108(11):4551-627. doi: 10.1021/cr800443h. Chem Rev. 2008. PMID: 19006398 Free PMC article. Review. No abstract available.
References
-
- Volcani B E. In: Silicon and Siliceous Structures in Biological Systems. Simpson T L, Volcani B E, editors. New York: Springer; 1981. pp. 157–201.
-
- Voronkov M G. In: Biochemistry of Silicon and Related Problems. Benz G, Lindqvist I, editors. New York: Plenum; 1997. pp. 395–434.
-
- DeLaRocha C L, Brzezinski M A, DeNiro M J. Geochim Cosmochim Acta. 1997;61:5051–5056.
-
- Hecky R E, Mopper K, Kilham P, Degens E T. Mar Biol (Berlin) 1973;19:323–331.
-
- Swift D M, Wheeler A P. Phycology. 1992;28:209–213.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

