Antitumor activity of mannan-binding protein in vivo as revealed by a virus expression system: mannan-binding proteindependent cell-mediated cytotoxicity
- PMID: 9892640
- PMCID: PMC15143
- DOI: 10.1073/pnas.96.2.371
Antitumor activity of mannan-binding protein in vivo as revealed by a virus expression system: mannan-binding proteindependent cell-mediated cytotoxicity
Abstract
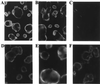
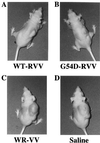
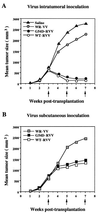
Mannan-binding protein (MBP), a Ca2+-dependent mammalian lectin specific for mannose and N-acetylglucosamine, is an important serum component associated with innate immunity. MBP activates complement and functions as a direct opsonin on binding to mannooligosaccharide-bearing pathogens. We have found that MBP recognizes and binds specifically to oligosaccharide ligands expressed on the surfaces of a human colorectal carcinoma. Interestingly, the recombinant vaccinia virus carrying human MBP gene was demonstrated to possess a potent growth-inhibiting activity against human colorectal carcinoma cells transplanted in KSN nude mice when administered by intratumoral or subcutaneous injection. Moreover, a significant prolongation of life span of tumor-bearing mice resulted from the treatment. This effect appears to be a consequence of local production of MBP. Unexpectedly, the mutant MBP, which had essentially no complement-activating activity, was nearly as active as wild-type MBP. These results indicated that MBP has a previously undescribed cytotoxic activity, which we propose to term MBP-dependent cell-mediated cytotoxicity(MDCC). In addition, this study provides a model for the development of an effective and specific host defense factor for cancer gene therapy.
Figures


References
-
- Kawasaki N, Kawasaki T, Yamashina I. J Biochem (Tokyo) 1983;94:937–947. - PubMed
-
- Kurata H, Sannoh T, Kozutsumi Y, Yokota Y, Kawasaki T. J Biochem (Tokyo) 1994;115:1148–1154. - PubMed
-
- Drickamer K, Dordal M S, Reynolds L. J Biol Chem. 1986;261:6878–6887. - PubMed
-
- Thiel S, Vorup-Jensen T, Stover C M, Schwaeble W, Laursen S B, Poulsen K, Willis A C, Eggleton P, Hansen S, Holmskov U, et al. Nature (London) 1997;386:506–510. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

