A direct role for arrestins in desensitization of the luteinizing hormone/choriogonadotropin receptor in porcine ovarian follicular membranes
- PMID: 9892661
- PMCID: PMC15164
- DOI: 10.1073/pnas.96.2.493
A direct role for arrestins in desensitization of the luteinizing hormone/choriogonadotropin receptor in porcine ovarian follicular membranes
Abstract
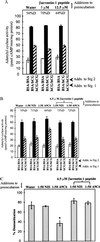
The luteinizing hormone/choriogonadotropin (LH/CG) receptor (R) is a heptahelical R that, upon agonist binding, activates the stimulatory guanine nucleotide-binding protein (Gs) and the downstream effector adenylyl cyclase (AC). Like other G protein-coupled Rs, the LH/CG R subsequently exhibits reduced agonist-dependent effector activity, or desensitization, in response to saturating agonist. Unlike desensitization of many other G protein-coupled Rs, the in vivo desensitization response of LH/CG R-stimulated AC activity of ovarian follicles to the preovulatory surge of LH can be mimicked under cell-free conditions. Based on evidence that porcine ovarian follicular membranes unexpectedly contained beta-arrestin-1, the role of arrestins in desensitization of the LH/CG R was investigated. Results showed that neutralizing arrestin antibodies blocked the development of desensitization and that desensitization was rescued with a synthetic peptide corresponding to the antibody-binding epitope on beta-arrestin-1. These results suggest that endogenous beta-arrestin-1 participates in agonist-dependent desensitization of the LH/CG R. Addition of recombinant purified beta-arrestin-1 mimicked human chorionic gonadotrophin to promote desensitization of human chorionic gonadotrophin-stimulated AC activity, in the presence of the ATP phosphorylation antagonist adenylyl-imidodiphosphate, with an ED50 of approximately 0.1 nM. Increased levels of an 87-kDa protein reactive with glycoprotein hormone R-reactive antibody, consistent with the LH/CG R, coimmunoprecipitated with follicular membrane beta-arrestin-1 in response to LH/CG R activation compared with unactivated R. Taken together, these results show that ovarian follicles contain membrane-associated beta-arrestin-1, that beta-arrestin-1 participates in agonist-dependent desensitization of the LH/CG R, and that the trigger for beta-arrestin-1 binding to the LH/CG R appears to be R activation.
Figures




Similar articles
-
beta-arrestin-dependent desensitization of luteinizing hormone/choriogonadotropin receptor is prevented by a synthetic peptide corresponding to the third intracellular loop of the receptor.J Biol Chem. 1999 May 7;274(19):12984-9. doi: 10.1074/jbc.274.19.12984. J Biol Chem. 1999. PMID: 10224047
-
The ADP ribosylation factor nucleotide exchange factor ARNO promotes beta-arrestin release necessary for luteinizing hormone/choriogonadotropin receptor desensitization.Proc Natl Acad Sci U S A. 2000 May 23;97(11):5901-6. doi: 10.1073/pnas.100127097. Proc Natl Acad Sci U S A. 2000. PMID: 10811902 Free PMC article.
-
Roles of Gi and Gq/11 in mediating desensitization of the luteinizing hormone/choriogonadotropin receptor in porcine ovarian follicular membranes.Endocrinology. 1999 Apr;140(4):1612-21. doi: 10.1210/endo.140.4.6657. Endocrinology. 1999. PMID: 10098495
-
ARF6: a newly appreciated player in G protein-coupled receptor desensitization.FEBS Lett. 2002 Jun 19;521(1-3):3-8. doi: 10.1016/s0014-5793(02)02822-3. FEBS Lett. 2002. PMID: 12067715 Review.
-
β-arrestins and biased signaling in gonadotropin receptors.Minerva Ginecol. 2018 Oct;70(5):525-538. doi: 10.23736/S0026-4784.18.04272-7. Epub 2018 Jul 10. Minerva Ginecol. 2018. PMID: 29999287 Review.
Cited by
-
Multifaceted roles of beta-arrestins in the regulation of seven-membrane-spanning receptor trafficking and signalling.Biochem J. 2003 Nov 1;375(Pt 3):503-15. doi: 10.1042/BJ20031076. Biochem J. 2003. PMID: 12959637 Free PMC article. Review.
-
Interaction of a fragment of the cannabinoid CB1 receptor C-terminus with arrestin-2.FEBS Lett. 2007 Oct 16;581(25):5009-16. doi: 10.1016/j.febslet.2007.09.030. Epub 2007 Sep 24. FEBS Lett. 2007. PMID: 17910957 Free PMC article.
-
Binding between a distal C-terminus fragment of cannabinoid receptor 1 and arrestin-2.Biochemistry. 2011 Mar 29;50(12):2223-34. doi: 10.1021/bi1018144. Epub 2011 Feb 28. Biochemistry. 2011. PMID: 21306178 Free PMC article.
-
Noncanonical GPCR signaling arising from a PTH receptor-arrestin-Gβγ complex.Proc Natl Acad Sci U S A. 2013 Jan 22;110(4):1530-5. doi: 10.1073/pnas.1205756110. Epub 2013 Jan 7. Proc Natl Acad Sci U S A. 2013. PMID: 23297229 Free PMC article.
-
Regulation of lutropin circulatory half-life by the mannose/N-acetylgalactosamine-4-SO4 receptor is critical for implantation in vivo.J Clin Invest. 2002 Jan;109(2):269-76. doi: 10.1172/JCI13997. J Clin Invest. 2002. PMID: 11805139 Free PMC article.
References
-
- Dohlman H G, Thorner J, Caron M G, Lefkowitz R J. Annu Rev Biochem. 1991;60:653–688. - PubMed
-
- Guderman T, Birnbaumer M, Birnbaumer L. J Biol Chem. 1992;267:4479–4488. - PubMed
-
- Loosfelt H, Misrahi M, Atger M, Salesse R, Vuhai-Luuthi M T, Jolivet A, Guiochon-Mantel A, Sar S, Jallal B, Garnier J, Milgrom E. Science. 1989;245:525–528. - PubMed
-
- McFarland K C, Sprengel R, Phillips H S, Kohler M, Rosembilt N, Nikolics K, Segaloff D L, Seeburg P H. Science. 1989;245:494–499. - PubMed
-
- Hausdorff W P, Caron M G, Lefkowitz R J. FASEB J. 1990;4:2881–2889. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

