Synergistic activities of multiple phosphotyrosine residues mediate full signaling from the Drosophila Torso receptor tyrosine kinase
- PMID: 9892666
- PMCID: PMC15169
- DOI: 10.1073/pnas.96.2.523
Synergistic activities of multiple phosphotyrosine residues mediate full signaling from the Drosophila Torso receptor tyrosine kinase
Abstract
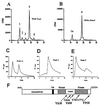
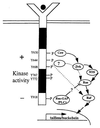
Here, we identify four tyrosine residues (Y644, Y698, Y767, and Y772) that become phosphorylated after activation of the Torso (Tor) receptor tyrosine kinase. Previously, we characterized phosphotyrosine sites (P-Y630 and P-Y918). Of the six P-Y sites identified, three (Y630, Y644, and Y698) are located in the kinase domain insert region, one (Y918) is located in the C-terminal tail region, and two (Y767 and Y772) are located in the activation loop of the kinase domain. To investigate the function of each P-Y residue in Tor signaling, we have generated transgenic Drosophila embryos expressing mutant Tor receptors containing either single or multiple tyrosine to phenylalanine substitutions. Single P-Y mutations were found to have either positive, negative, or no effect on the signaling activity of the receptor. Elimination of all P-Y sites within the kinase insert region resulted in the complete loss of receptor function, indicating that some combination of these sites is necessary for Tor signaling. Mutation of the C-terminal P-Y918 site revealed that this site is responsible for negative signaling or down-regulation of receptor activity. Mutation of the P-Y sites in the kinase domain activation loop demonstrated that these sites are essential for enzymatic activity. Our analysis provides a detailed in vivo example of the extent of cooperativity between P-Y residues in transducing the signal received by a receptor tyrosine kinase and in vivo data demonstrating the function of P-Y residues in the activation loop of the kinase domain.
Figures


Similar articles
-
Functions and mechanisms of receptor tyrosine kinase Torso signaling: lessons from Drosophila embryonic terminal development.Dev Dyn. 2005 Mar;232(3):656-72. doi: 10.1002/dvdy.20295. Dev Dyn. 2005. PMID: 15704136 Free PMC article. Review.
-
Drosophila terminal structure development is regulated by the compensatory activities of positive and negative phosphotyrosine signaling sites on the Torso RTK.Genes Dev. 1996 Mar 1;10(5):566-77. doi: 10.1101/gad.10.5.566. Genes Dev. 1996. PMID: 8598287
-
Opposing actions of CSW and RasGAP modulate the strength of Torso RTK signaling in the Drosophila terminal pathway.Mol Cell. 1998 Dec;2(6):719-27. doi: 10.1016/s1097-2765(00)80287-7. Mol Cell. 1998. PMID: 9885560
-
A conserved amino-terminal Shc domain binds to phosphotyrosine motifs in activated receptors and phosphopeptides.Curr Biol. 1995 Apr 1;5(4):404-12. doi: 10.1016/s0960-9822(95)00081-9. Curr Biol. 1995. PMID: 7542991
-
The torso pathway in Drosophila: a model system to study receptor tyrosine kinase signal transduction.Dev Suppl. 1993:47-56. Dev Suppl. 1993. PMID: 8049487 Review.
Cited by
-
Generation and purification of highly specific antibodies for detecting post-translationally modified proteins in vivo.Nat Protoc. 2014 Feb;9(2):375-95. doi: 10.1038/nprot.2014.017. Epub 2014 Jan 23. Nat Protoc. 2014. PMID: 24457330 Free PMC article.
-
Functions and mechanisms of receptor tyrosine kinase Torso signaling: lessons from Drosophila embryonic terminal development.Dev Dyn. 2005 Mar;232(3):656-72. doi: 10.1002/dvdy.20295. Dev Dyn. 2005. PMID: 15704136 Free PMC article. Review.
-
Ligand-dependent responses of the silkworm prothoracicotropic hormone receptor, Torso, are maintained by unusual intermolecular disulfide bridges in the transmembrane region.Sci Rep. 2016 Mar 1;6:22437. doi: 10.1038/srep22437. Sci Rep. 2016. PMID: 26928300 Free PMC article.
References
-
- Dickson B, Hafen E. Curr Opin Genet Dev. 1994;4:64–70. - PubMed
-
- Lu X, Chou T-B, Williams N, Robert T, Perrimon N. Genes Dev. 1993;7:621–632. - PubMed
-
- Ullrich A, Schlessinger J. Cell. 1990;61:203–212. - PubMed
-
- Heldin C H. Cell. 1995;80:2132–223. - PubMed
-
- Cantley L C, Auger K R, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S. Cell. 1991;64:281–302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

