MyoR: a muscle-restricted basic helix-loop-helix transcription factor that antagonizes the actions of MyoD
- PMID: 9892671
- PMCID: PMC15174
- DOI: 10.1073/pnas.96.2.552
MyoR: a muscle-restricted basic helix-loop-helix transcription factor that antagonizes the actions of MyoD
Abstract
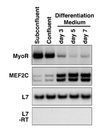
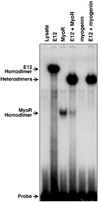
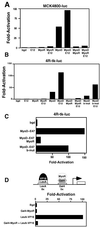
Skeletal muscle development is controlled by a family of muscle-specific basic helix-loop-helix (bHLH) transcription factors that activate muscle genes by binding E-boxes (CANNTG) as heterodimers with ubiquitous bHLH proteins, called E proteins. Myogenic bHLH factors are expressed in proliferating undifferentiated myoblasts, but they do not initiate myogenesis until myoblasts exit the cell cycle. We describe a bHLH protein, MyoR (for myogenic repressor), that is expressed in undifferentiated myoblasts in culture and is down-regulated during differentiation. MyoR is also expressed specifically in the skeletal muscle lineage between days 10.5 and 16.5 of mouse embryogenesis and down-regulated thereafter during the period of secondary myogenesis. MyoR forms heterodimers with E proteins that bind the same DNA sequence as myogenic bHLH/E protein heterodimers, but MyoR acts as a potent transcriptional repressor that blocks myogenesis and activation of E-box-dependent muscle genes. These results suggest a role for MyoR as a lineage-restricted transcriptional repressor of the muscle differentiation program.
Figures


References
-
- Yun S, Wold B J. Curr Opin Cell Biol. 1996;8:877–889. - PubMed
-
- Molkentin J, Olson E N. Curr Opin Genet Dev. 1996;6:445–453. - PubMed
-
- Rudnicki M A, Schnegelsberg P N J, Stead R H, Braun T, Arnold H H, Jaenisch R. Cell. 1993;71:1351–1359. - PubMed
-
- Hasty P, Bradley A, Morris J H, Venuti J M, Olson E N, Klein W H. Nature (London) 1993;364:501–506. - PubMed
-
- Nabeshima Y K, Hanaoka K, Hayasaka M, Esumi S, Li S, Nonaka I, Nabeshima Y. Nature (London) 1993;364:532–535. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

