Nitric oxide synthase in cardiac sarcoplasmic reticulum
- PMID: 9892689
- PMCID: PMC15192
- DOI: 10.1073/pnas.96.2.657
Nitric oxide synthase in cardiac sarcoplasmic reticulum
Abstract
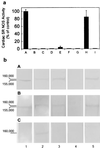
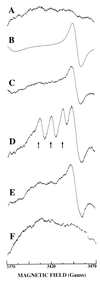
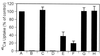
NO. is a free radical that modulates heart function and metabolism. We report that a neuronal-type NO synthase (NOS) is located on cardiac sarcoplasmic reticulum (SR) membrane vesicles and that endogenous NO. produced by SR-associated NOS inhibits SR Ca2+ uptake. Ca2+-dependent biochemical conversion of L-arginine to L-citrulline was observed from isolated rabbit cardiac SR vesicles in the presence of NOS substrates and cofactors. Endogenous NO. was generated from the vesicles and detected by electron paramagnetic resonance spin-trapping measurements. Immunoelectron microscopy demonstrated labeling of cardiac SR vesicles by using anti-neuronal NOS (nNOS), but not anti-endothelial NOS (eNOS) or anti-inducible NOS (iNOS) antibodies, whereas skeletal muscle SR vesicles had no nNOS immunoreactivity. The nNOS immunoreactivity also displayed a pattern consistent with SR localization in confocal micrographs of sections of human myocardium. Western blotting demonstrated that cardiac SR NOS is larger than brain NOS (160 vs. 155 kDa). No immunodetection was observed in cardiac SR vesicles from nNOS knockout mice or with an anti-nNOS mu antibody, suggesting the possibility of a new nNOS-type isoform. 45Ca uptake by cardiac SR vesicles, catalyzed by Ca2+-ATPase, was inhibited by NO. produced endogenously from cardiac SR NOS, and 7-nitroindazole, a selective nNOS inhibitor, completely prevented this inhibition. These results suggest that a cardiac muscle nNOS isoform is located on SR of cardiac myocytes, where it may respond to intracellular Ca2+ concentration and modulate SR Ca2+ ion active transport in the heart.
Figures

References
-
- Nakane M, Schmidt H H, Pollock J S, Forstermann U, Murad F. FEBS Lett. 1993;316:175–180. - PubMed
-
- Kobzik L, Reid M B, Bredt D S, Stamler J S. Nature (London) 1994;372:546–548. - PubMed
-
- Brenman J E, Chao D S, Xia H, Aldape K, Bredt D S. Cell. 1995;82:743–752. - PubMed
-
- Silvagno F, Xia H, Bredt D S. J Biol Chem. 1996;271:11204–11208. - PubMed
-
- Kelly R A, Balligad J L, Smith T W. Circ Res. 1996;79:363–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

