CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue
- PMID: 9892690
- PMCID: PMC15193
- DOI: 10.1073/pnas.96.2.663
CXCR4 utilization is sufficient to trigger CD4+ T cell depletion in HIV-1-infected human lymphoid tissue
Abstract
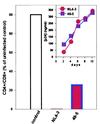
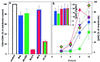
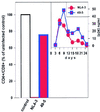
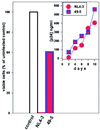
The human chemokine receptors CCR5 and CXCR4 have emerged as the predominant cofactors, along with CD4, for cellular entry of HIV-1 in vivo whereas the contribution of other chemokine receptors to HIV disease has not been yet determined. CCR5-specific (R5) viruses predominate during primary HIV-1 infection whereas viruses with specificity for CXCR4 (R5/X4 or X4 viruses) often emerge in late stages of HIV disease. The evolution of X4 viruses is associated with a rapid decline in CD4+ T cells, although a causative relationship between viral tropism and CD4+ T cell depletion has not yet been proven. To rigorously test this relationship, we assessed CD4+ T cell depletion in suspensions of human peripheral blood mononuclear cells and in explants of human lymphoid tissue on exposure to paired viruses that are genetically identical (isogenic) except for select envelope determinants specifying reciprocal tropism for CXCR4 or CCR5. In both systems, X4 HIV-1 massively depleted CD4+ lymphocytes whereas matched R5 viruses depleted such cells only mildly despite comparable viral replication kinetics. These findings demonstrate that the coreceptor specificities of HIV-1 are a causal factor in CD4+ T cell depletion ex vivo and strongly support the hypothesis that the evolution of viral envelope leading to usage of CXCR4 in vivo accelerates loss of CD4+ T cells, causing immunodeficiency.
Figures

Similar articles
-
Differential effects of R5 and X4 human immunodeficiency virus type 1 infection on CD4+ cell proliferation and activation.J Gen Virol. 2005 Apr;86(Pt 4):1171-1179. doi: 10.1099/vir.0.80674-0. J Gen Virol. 2005. PMID: 15784911
-
V3 loop-determined coreceptor preference dictates the dynamics of CD4+-T-cell loss in simian-human immunodeficiency virus-infected macaques.J Virol. 2005 Oct;79(19):12296-303. doi: 10.1128/JVI.79.19.12296-12303.2005. J Virol. 2005. PMID: 16160156 Free PMC article.
-
Envelope-dependent restriction of human immunodeficiency virus type 1 spreading in CD4(+) T lymphocytes: R5 but not X4 viruses replicate in the absence of T-cell receptor restimulation.J Virol. 1999 Sep;73(9):7515-23. doi: 10.1128/JVI.73.9.7515-7523.1999. J Virol. 1999. PMID: 10438841 Free PMC article.
-
Relationships Between HIV-Mediated Chemokine Coreceptor Signaling, Cofilin Hyperactivation, Viral Tropism Switch and HIV-Mediated CD4 Depletion.Curr HIV Res. 2019;17(6):388-396. doi: 10.2174/1570162X17666191106112018. Curr HIV Res. 2019. PMID: 31702526 Review.
-
The role of viral coreceptors and enhanced macrophage tropism in human immunodeficiency virus type 1 disease progression.Sex Health. 2004;1(1):23-34. doi: 10.1071/sh03006. Sex Health. 2004. PMID: 16335478 Review.
Cited by
-
Preferential coreceptor utilization and cytopathicity by dual-tropic HIV-1 in human lymphoid tissue ex vivo.J Clin Invest. 1999 Sep;104(5):R7-R11. doi: 10.1172/JCI7403. J Clin Invest. 1999. PMID: 10487781 Free PMC article.
-
Adding new dimensions: towards an integrative understanding of HIV-1 spread.Nat Rev Microbiol. 2014 Aug;12(8):563-74. doi: 10.1038/nrmicro3309. Nat Rev Microbiol. 2014. PMID: 25029025 Free PMC article. Review.
-
Mucosal Responses to Zika Virus Infection in Cynomolgus Macaques.Pathogens. 2022 Sep 12;11(9):1033. doi: 10.3390/pathogens11091033. Pathogens. 2022. PMID: 36145466 Free PMC article.
-
Dual role of prostratin in inhibition of infection and reactivation of human immunodeficiency virus from latency in primary blood lymphocytes and lymphoid tissue.J Virol. 2004 Oct;78(19):10507-15. doi: 10.1128/JVI.78.19.10507-10515.2004. J Virol. 2004. PMID: 15367617 Free PMC article.
-
Human immunodeficiency virus type 1 pathogenesis in SCID-hu mice correlates with syncytium-inducing phenotype and viral replication.J Virol. 2000 Apr;74(7):3196-204. doi: 10.1128/jvi.74.7.3196-3204.2000. J Virol. 2000. PMID: 10708436 Free PMC article.
References
-
- Freed E O, Martin M A. J Biol Chem. 1995;270:23883–23886. - PubMed
-
- Feng Y, Broder C C, Kennedy P E, Berger E A. Science. 1996;272:872–877. - PubMed
-
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. Science. 1996;272:1955–1958. - PubMed
-
- Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, et al. Cell. 1996;85:1135–1148. - PubMed
-
- Deng H, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, Di Marzio P, Marmon S, Sutton R E, Hill C M, et al. Nature (London) 1996;381:661–666. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

