Expression of cell adhesion molecules and vascular endothelial growth factor in experimental choroidal neovascularisation in the rat
- PMID: 9893599
- PMCID: PMC1722761
- DOI: 10.1136/bjo.82.9.1063
Expression of cell adhesion molecules and vascular endothelial growth factor in experimental choroidal neovascularisation in the rat
Abstract
Aims: To investigate the longevity and reproducibility of choroidal neovascularisation (CNV) induced by krypton laser photocoagulation in the rat. The presence of cell adhesion molecules (CAMs) and vascular endothelial growth factor (VEGF) during the development of CNV was also studied.
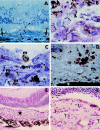
Methods: 67 pigmented rats underwent retinal photocoagulation by krypton laser. The eyes were examined by either single or serial fluorescein angiography at 3 days, 1, 2-3, 4-5, 7-8, and 12 weeks post photocoagulation. The expression of CAMs (ICAM-1, E-selectin, and CD44) and VEGF post photocoagulation was studied by immunohistochemistry.
Results: CNV related fluorescein leakage appeared in 46.4% of 766 laser spots delivered to the 58 eyes that were tested at 2-3 weeks post treatment. The ratio of hyperfluorescent laser sites did not change significantly at 8 weeks post laser. The number of leaky spots was independent of the total number of lesions delivered to each eye (at 2-3 weeks post laser 10-15 spots/eye: 44% and 25-30 spots/eye: 49%; t = 0.7673; p = 0.3903). Nine eyes were followed by serial angiography between 2 and 12 weeks. The laser spots with fluorescein leakage at 2 weeks (51.5%) remained leaky at 12 weeks (51.5%). Histopathologically, macrophage accumulation peaked at 5 days and CNV was firstly observed at 1 week post photocoagulation. ICAM-1, E-selectin, CD44, and VEGF were maximally induced at 3-5 days post laser photocoagulation, and were localised to RPE, choroidal vascular endothelial, and inflammatory cells. VEGF was also detected in intravascular leucocytes at the sites of laser lesions.
Conclusions: These studies demonstrated that krypton laser photocoagulation can be successfully used to produce lesions similar to those of human CNV. The response induced remained present for an extended period of time (12 weeks), thus offering a potential model to screen candidate CNV inhibitory agents. In addition, it is proposed that the expression of ICAM-1, E-selectin, CD44, and VEGF before new vessel formation might be linked to the initiation of CNV.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
