Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation
- PMID: 9895382
- PMCID: PMC1727381
- DOI: 10.1136/gut.44.2.226
Comparison of a low dose polyethylene glycol electrolyte solution with lactulose for treatment of chronic constipation
Abstract
Background: Polyethylene glycol (PEG) 3350 is a non-absorbable, non-metabolised osmotic agent used in lavage solutions for gut cleansing.
Aims: To compare the efficacy of PEG and lactulose in chronic constipation.
Methods: A total of 115 patients with chronic constipation entered a multicentre, randomised, comparative trial. They initially received two sachets containing either PEG (13 g/sachet) or lactulose (10 g/sachet) and were given an option to change the dose to one or three sachets/day, depending on response.
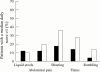
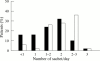
Results: Ninety nine patients completed the trial. After four weeks, patients in the PEG group (n=50) had a higher number of stools and a lower median daily score for straining at stool than patients in the lactulose group (n=49). Overall improvement was greater in the PEG group. Clinical tolerance was similar in the two groups, but flatus was less frequently reported in the PEG group. The mean number of liquid stools was higher in the PEG group but the difference was significant only for the first two weeks. There were no serious adverse events and no significant change in laboratory tests in either group. At the end of the study, the number of sachets used by the patients was 1.6 (0.7)/day in the PEG group and 2.1 (0.7)/day in the lactulose group. Sixty one patients completed a further two months open study of one to three sachets PEG daily; there was no loss of efficacy and no serious toxicity.
Conclusion: Low dose PEG 3350 was more effective than lactulose and better tolerated.
Figures
Similar articles
-
PEG 3350 (Transipeg) versus lactulose in the treatment of childhood functional constipation: a double blind, randomised, controlled, multicentre trial.Gut. 2004 Nov;53(11):1590-4. doi: 10.1136/gut.2004.043620. Gut. 2004. PMID: 15479678 Free PMC article. Clinical Trial.
-
Cochrane Review: Osmotic and stimulant laxatives for the management of childhood constipation (Review).Evid Based Child Health. 2013 Jan;8(1):57-109. doi: 10.1002/ebch.1893. Evid Based Child Health. 2013. PMID: 23878124 Review.
-
Tolerance and Long-Term Efficacy of Polyethylene Glycol 4000 (Forlax®) Compared to Lactulose in Elderly Patients with Chronic Constipation.J Nutr Health Aging. 2017;21(4):429-439. doi: 10.1007/s12603-016-0762-6. J Nutr Health Aging. 2017. PMID: 28346570 Clinical Trial.
-
Application of Oral Lactulose in Combination With Polyethylene Glycol Electrolyte Powder for Colonoscopy Bowel Preparation in Patients With Constipation.Am J Ther. 2016 Jul-Aug;23(4):e1020-4. doi: 10.1097/MJT.0000000000000351. Am J Ther. 2016. PMID: 26658804 Clinical Trial.
-
Polyethylene glycol combined with lactulose has better efficacy than polyethylene glycol alone in bowel preparation before colonoscopy: A meta-analysis.Clinics (Sao Paulo). 2023 Apr 3;78:100172. doi: 10.1016/j.clinsp.2023.100172. eCollection 2023. Clinics (Sao Paulo). 2023. PMID: 37019039 Free PMC article. Review.
Cited by
-
Appropriate Use of Laxatives in the Older Person.Drugs Aging. 2019 Nov;36(11):999-1005. doi: 10.1007/s40266-019-00701-9. Drugs Aging. 2019. PMID: 31478168 Review.
-
Action Mode of Gut Motility, Fluid and Electrolyte Transport in Chronic Constipation.Front Pharmacol. 2021 Jul 27;12:630249. doi: 10.3389/fphar.2021.630249. eCollection 2021. Front Pharmacol. 2021. PMID: 34385914 Free PMC article. Review.
-
Effects of Fructo-Oligosaccharide Supplementation on Constipation in Elderly Continuous Ambulatory Peritoneal Dialysis Patients.Perit Dial Int. 2016 Jan-Feb;36(1):60-6. doi: 10.3747/pdi.2014.00015. Epub 2014 Oct 7. Perit Dial Int. 2016. PMID: 25292404 Free PMC article. Clinical Trial.
-
Opioid-Induced Constipation and Bowel Dysfunction: A Clinical Guideline.Pain Med. 2017 Oct 1;18(10):1837-1863. doi: 10.1093/pm/pnw255. Pain Med. 2017. PMID: 28034973 Free PMC article. Review.
-
Treatment of constipation in older people.CMAJ. 2013 May 14;185(8):663-70. doi: 10.1503/cmaj.120819. Epub 2013 Jan 28. CMAJ. 2013. PMID: 23359042 Free PMC article. Review. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
